所有图片(1)
About This Item
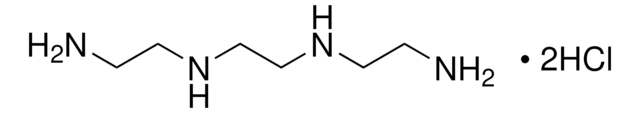
线性分子式:
[-SC(CH3)2CH(NH2)CO2H]2
CAS号:
分子量:
296.41
Beilstein:
4461521
EC號碼:
MDL號碼:
分類程式碼代碼:
12352116
PubChem物質ID:
NACRES:
NA.22
推荐产品
产品名称
D-青霉胺二硫化物, 97%
品質等級
化驗
97%
形狀
powder or crystals
光學活性
[α]25/D −75°, c = 1 in 1 M NaOH
反應適用性
reaction type: solution phase peptide synthesis
顏色
white
mp
204 °C (dec.) (lit.)
應用
cell analysis
SMILES 字串
CC(C)(SSC(C)(C)[C@@H](N)C(O)=O)[C@@H](N)C(O)=O
InChI
1S/C10H20N2O4S2/c1-9(2,5(11)7(13)14)17-18-10(3,4)6(12)8(15)16/h5-6H,11-12H2,1-4H3,(H,13,14)(H,15,16)/t5-,6-/m0/s1
InChI 密鑰
POYPKGFSZHXASD-WDSKDSINSA-N
相关类别
應用
Used in pharmacological studies of:
- Molecules dermatomyositis activity
- Antimelanoma activity of apoptogenic carbonyl scavengers
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Fabrizio Apruzzese et al.
Annali di chimica, 94(1-2), 45-56 (2004-05-15)
D-penicillamine disulfide (PNS) shows protolytic properties and is able to form complexes with cations, because it has two aminic groups and two carboxylic groups. The four protonation constants of its deprotonated species were determined by means of electromotive force (e.m.f.)
[Determination of D-penicillamine and its metabolites in blood and urine].
K Kyogoku et al.
Yakugaku zasshi : Journal of the Pharmaceutical Society of Japan, 102(4), 322-327 (1982-04-01)
Determination of penicillamine in encapsulated formulations by high-performance liquid chromatography.
S Biffar et al.
Journal of chromatography, 318(2), 404-407 (1985-01-18)
Amit K Galande et al.
Biopolymers, 71(5), 534-551 (2003-11-25)
A recently rediscovered reaction of base-assisted lanthionine formation has been applied to several systems of disulfide-bridged peptides. In addition to previously described nonapeptides consisting of i, i+3 cystine linkages, the reaction has now been extended to systems consisting of shorter
Anshul Gupte et al.
Journal of inorganic biochemistry, 101(4), 594-602 (2007-02-06)
D-Penicillamine is a potent copper (Cu) chelating agent. D-Pen reduces Cu(II) to Cu(I) in the process of chelation while at the same time being oxidized to D-penicillamine disulfide. It has been proposed that hydrogen peroxide is generated during this process.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门