推荐产品
蒸汽密度
3 (vs air)
蒸汽壓力
1.5 mmHg ( 20 °C)
產品線
ReagentPlus®
化驗
≥99%
形狀
liquid
自燃溫度
851 °F
expl. lim.
16 %
折射率
n20/D 1.436 (lit.)
bp
204-205 °C (lit.)
mp
−45 °C (lit.)
密度
1.12 g/mL at 25 °C (lit.)
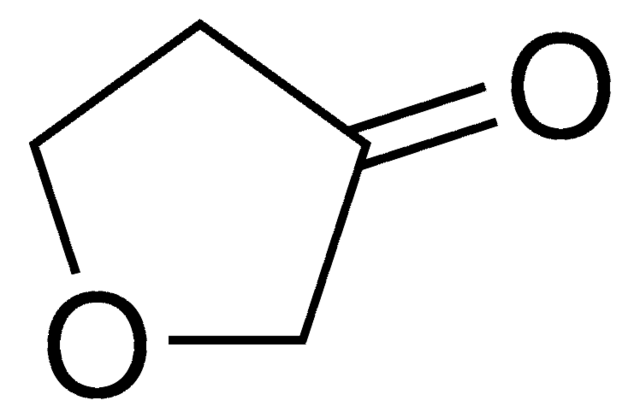
SMILES 字串
O=C1CCCO1
InChI
1S/C4H6O2/c5-4-2-1-3-6-4/h1-3H2
InChI 密鑰
YEJRWHAVMIAJKC-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
用于引入3-羧丙基侧链。用作电池和电容器中电解质溶液的组成成分。
生化/生理作用
γ-羟基丁酸(GHB)前体。 可通过阻断多巴胺能神经元的脉冲流,抑制多巴胺释放。 γ-丁内酯预处理可用于检测自身受体诱导的多巴胺释放。
法律資訊
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 4 Oral - Eye Dam. 1 - STOT SE 3
標靶器官
Central nervous system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 1
閃點(°F)
208.4 °F - closed cup
閃點(°C)
98 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Journal of Power Sources, 43, 195-195 (1993)
S Ohkawa et al.
Journal of medicinal chemistry, 34(1), 267-276 (1991-01-01)
A novel series of (3-pyridylmethyl)benzoquinone derivatives was molecular designed and synthesized for the dual purpose of inhibiting thromboxane A2 and leukotriene biosynthesis enzymes and scavenging active oxygen species (AOS). They were evaluated for inhibition of TXA2 synthase, inhibition of 5-lipoxygenase
Jagan N Thupari et al.
Proceedings of the National Academy of Sciences of the United States of America, 99(14), 9498-9502 (2002-06-13)
C75, a known inhibitor of fatty acid synthase is postulated to cause significant weight loss through decreased hypothalamic neuropeptide Y (NPY) production. Peripherally, C75, an alpha-methylene-gamma-butyrolactone, reduces adipose tissue and fatty liver, despite high levels of malonyl-CoA. To investigate this
Michael Seitz et al.
Current opinion in chemical biology, 9(3), 285-292 (2005-06-09)
Natural products containing a gamma-butyrolactone ring are abundant in nature; however, few general synthetic approaches to their stereoselective synthesis with broad structural variety are known. In this article, recent developments towards mono- and polycyclic gamma-butyrolactone natural products and analogs are
Michelle Wood et al.
Journal of chromatography. A, 1056(1-2), 83-90 (2004-12-15)
We have developed a rapid method that enables the simultaneous analysis of gamma-hydroxybutyrate (GHB) and its precursors, i.e. gamma-butyrolactone (GBL) and 1,4-butanediol (1,4-BD) in urine. The method comprised a simple dilution of the urine sample, followed by liquid chromatography-tandem mass
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门