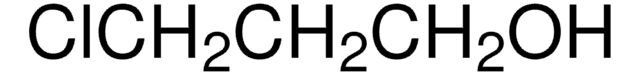推荐产品
化驗
98%
形狀
powder
mp
295 °C (dec.) (lit.)
SMILES 字串
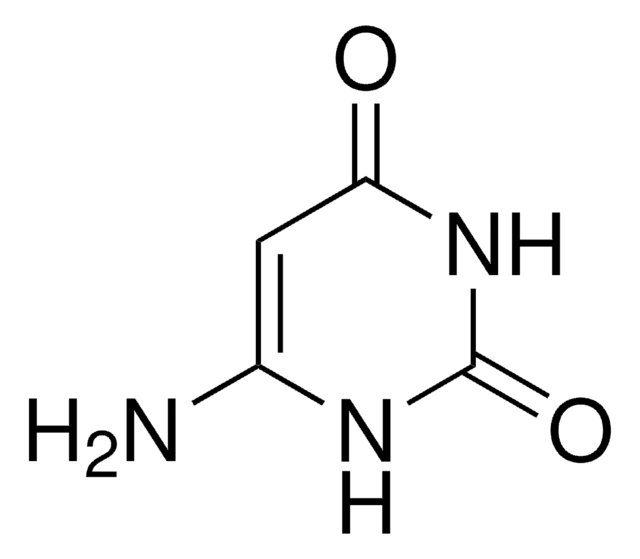
CN1C(N)=CC(=O)N(C)C1=O
InChI
1S/C6H9N3O2/c1-8-4(7)3-5(10)9(2)6(8)11/h3H,7H2,1-2H3
InChI 密鑰
VFGRNTYELNYSKJ-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Wafaa S Hamama et al.
Journal of advanced research, 4(2), 115-121 (2013-03-01)
The reaction of 6-amino-1,3-dimethylpyrimidine-2,4(1H,3H)-dione (1) as a binucleophile with primary aromatic or heterocyclic amines and formaldehyde or aromatic (heterocyclic) aldehydes in a molar ratio (1:1:2) gave the pyrimido[4,5-d]pyrimidin-2,4-dione ring systems 2-5. Treatment of 1 with diamines and formalin in molar
Javier Campanini-Salinas et al.
Molecules (Basel, Switzerland), 23(7) (2018-07-22)
A rapid emergence of resistant bacteria is occurring worldwide, endangering the efficacy of antibiotics and reducing the therapeutic arsenal available for treatment of infectious diseases. In the present study, we developed a new class of compounds with antibacterial activity obtained
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门