所有图片(1)
About This Item
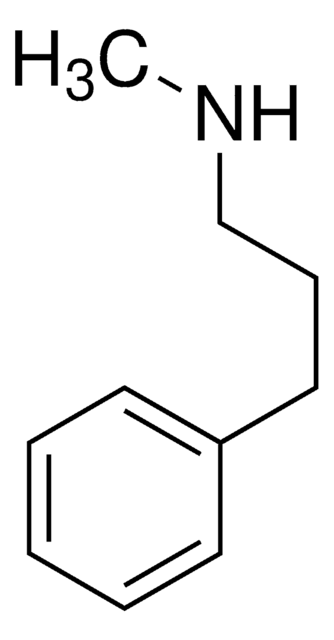
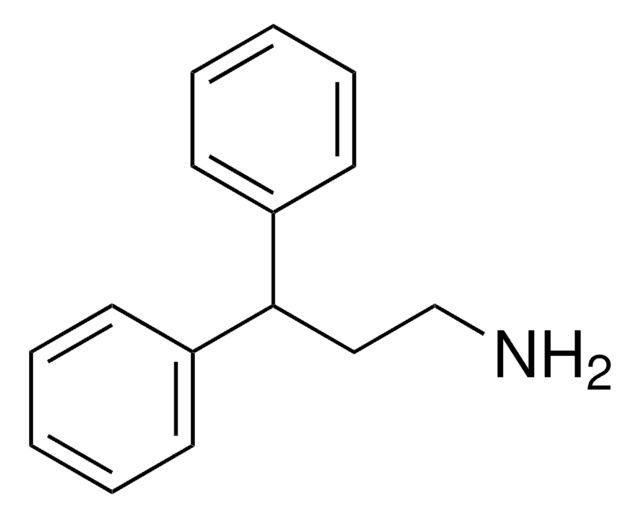
线性分子式:
C6H5CH2CH2CH(NH2)CH3
CAS号:
分子量:
149.23
Beilstein:
2413110
EC號碼:
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
品質等級
化驗
98%
形狀
liquid
折射率
n20/D 1.514 (lit.)
bp
228-232 °C (lit.)
密度
0.922 g/mL at 25 °C (lit.)
SMILES 字串
CC(N)CCc1ccccc1
InChI
1S/C10H15N/c1-9(11)7-8-10-5-3-2-4-6-10/h2-6,9H,7-8,11H2,1H3
InChI 密鑰
WECUIGDEWBNQJJ-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
訊號詞
Danger
危險分類
Acute Tox. 3 Dermal - Acute Tox. 3 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1B
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 2
閃點(°F)
208.0 °F
閃點(°C)
97.78 °C
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
其他客户在看
Maiko Kusano et al.
Forensic science international, 300, 125-135 (2019-05-20)
Trends in forensic toxicology show the advancement of rapid and sensitive analytical methods for qualitative and quantitative analysis of drugs of abuse. However, forensic toxicologists are continuously faced with the challenges of identifying and quantifying drug blood concentration while simultaneously
J Gal et al.
Research communications in chemical pathology and pharmacology, 62(1), 3-17 (1988-10-01)
Previous studies on the metabolic fate of antihypertensive agent labetalol in humans identified only conjugated metabolites of the drug and accounted for only a portion of the dose. In this study, urine samples obtained from three patients on chronic labetalol
R B Gilbert et al.
Journal of analytical toxicology, 19(2), 84-86 (1995-03-01)
A metabolite of labetalol that is responsible for previous reports of false-positive assays for amphetamines by thin-layer chromatography and immunoassay has been identified. The compound, 3-amino-1-phenylbutane (APB), an oxidative metabolite of labetalol, was initially identified in a patient's urine by
K Yeleswaram et al.
Drug metabolism and disposition: the biological fate of chemicals, 21(2), 284-292 (1993-03-01)
Labetalol causes significant maternal and fetal metabolic effects in pregnant sheep (Yeleswaram et al., J. Pharmacol. Exp. Ther. 262, 683-691 (1992)). This study was undertaken to investigate the contribution of skeletal muscles in the development of metabolic acidosis induced by
Hui Chen et al.
Journal of the American Chemical Society, 141(12), 4963-4971 (2019-03-06)
Enantiomerically pure chiral amines are of increasing value in the preparation of bioactive compounds, pharmaceuticals, and agrochemicals. ω-Transaminase (ω-TA) is an ideal catalyst for asymmetric amination because of its excellent enantioselectivity and wide substrate scope. To shift the equilibrium of
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持