推荐产品
方案
90%
表单
liquid
反应适用性
reaction type: click chemistry
折射率
n20/D 1.475
密度
1.167 g/mL at 25 °C
SMILES字符串
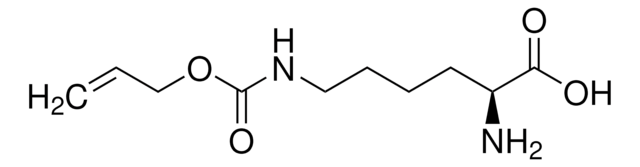
CC(C)(C)OC(=O)N[C@@H](CCCCNC(=O)OCC#C)C(O)=O
InChI
1S/C15H24N2O6/c1-5-10-22-13(20)16-9-7-6-8-11(12(18)19)17-14(21)23-15(2,3)4/h1,11H,6-10H2,2-4H3,(H,16,20)(H,17,21)(H,18,19)/t11-/m0/s1
InChI key
VHCJWKDEXGPNCX-NSHDSACASA-N
应用
Alkyne modified lysine that has been shown to be incorporated site-specifically into recombinant proteins.
其他客户在看
Synthesis of threefold glycosylated proteins using click chemistry and genetically encoded unnatural amino acids.
Emine Kaya et al.
Chembiochem : a European journal of chemical biology, 10(18), 2858-2861 (2009-11-10)
Duy P Nguyen et al.
Journal of the American Chemical Society, 131(25), 8720-8721 (2009-06-12)
We demonstrate that an orthogonal Methanosarcina barkeri MS pyrrolysyl-tRNA synthetase/tRNA(CUA) pair directs the efficient, site-specific incorporation of N6-[(2-propynyloxy)carbonyl]-L-lysine, containing a carbon-carbon triple bond, and N6-[(2-azidoethoxy)carbonyl]-L-lysine, containing an azido group, into recombinant proteins in Escherichia coli. Proteins containing the alkyne functional
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持