所有图片(3)
About This Item
经验公式(希尔记法):
C3H8N4
CAS号:
分子量:
100.12
MDL號碼:
分類程式碼代碼:
12352116
PubChem物質ID:
NACRES:
NA.22
推荐产品
品質等級
化驗
≥95%
形狀
liquid
反應適用性
reaction type: click chemistry
折射率
n20/D 1.471
密度
1.020 g/mL at 25 °C
儲存溫度
−20°C
SMILES 字串
NCCCN=[N+]=[N-]
InChI
1S/C3H8N4/c4-2-1-3-6-7-5/h1-4H2
InChI 密鑰
OYBOVXXFJYJYPC-UHFFFAOYSA-N
一般說明
3-叠氮基-1-丙胺可用于官能化以下物质:
- 含叠氮官能团的双羟甲基丙酸(双-MPA)单体,生成高代树枝状聚合物,
- 含叠氮基团可点击的四苯基卟啉锌支架,通过点击化学应用于光动力疗法。
應用
用于点击化学的胺修饰叠氮化物。
3-叠氮-1-丙胺可用于合成吡喃甘露糖苷树状大分子,用于研究多价碳水化合物-蛋白质相互作用。
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral - Flam. Liq. 3
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
140.0 °F
閃點(°C)
60 °C
其他客户在看
Xiaoqiang Chen et al.
Biomaterials, 122, 130-140 (2017-01-24)
The development of multifunctional reagents for simultaneous specific near-infrared (NIR) imaging and phototherapy of tumors is of great significance. This work describes the design of a cathepsin B-activated fluorescent probe (CyA-P-CyB) and its applications as an NIR imaging probe for
Anthony Angeli et al.
Chembiochem : a European journal of chemical biology, 18(11), 1036-1047 (2017-03-21)
Lectin A (LecA) from Pseudomonas aeruginosa is an established virulence factor. Glycoclusters that target LecA and are able to compete with human glycoconjugates present on epithelial cells are promising candidates to treat P. aeruginosa infection. A family of 32 glycodendrimers of generation 0
Mariano Ortega-Muñoz et al.
Nanoscale, 11(16), 7850-7856 (2019-04-10)
Activated carbon nanodots functionalized with acid anhydride groups (AA-CNDs) are prepared by one-pot water-free green thermolysis of citric acid. As a proof of concept of their capabilities as appealing and versatile platforms for accessing engineering nanoconstructs, the as-prepared AA-CNDs have
Dual display of proteins on the yeast cell surface simplifies quantification of binding interactions and enzymatic bioconjugation reactions.
Lim S, et al.
Biotechnology Journal, 12(5) (2017)
Two-dimensional ultrafast vibrational spectroscopy of azides in ionic liquids reveals solute-specific solvation
Dutta S, et al.
Physical Chemistry Chemical Physics, 17(40), 26575-26579 (2015)
商品
Drug discovery process by utilizing chemistry reaction of Cu(I)-catalyzed Huisgen 1,3-dipolar cycloaddition of terminal alkynes with organoazides to yield 1,4-disubstituted 1,2,3-triazoles.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门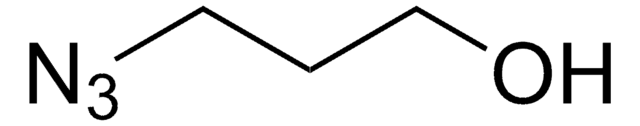


![2-[2-(2-氨基乙氧基)乙氧基]乙醇 溶液 ~0.5 M in tert-butyl methyl ether](/deepweb/assets/sigmaaldrich/product/structures/374/007/eea7ca74-41e4-4aac-af71-c93c37ec0a5a/640/eea7ca74-41e4-4aac-af71-c93c37ec0a5a.png)