所有图片(1)
About This Item
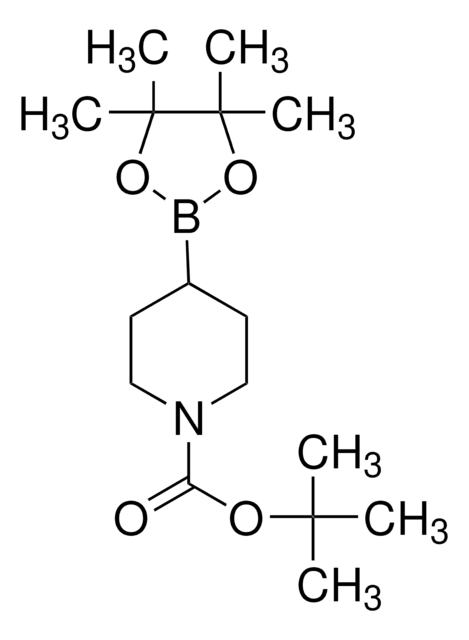
经验公式(希尔记法):
C16H28BNO4
CAS号:
分子量:
309.21
MDL號碼:
分類程式碼代碼:
12352103
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
95%
形狀
powder
mp
100-114 °C
SMILES 字串
CC(C)(C)OC(=O)N1CCC(=CC1)B2OC(C)(C)C(C)(C)O2
InChI
1S/C16H28BNO4/c1-14(2,3)20-13(19)18-10-8-12(9-11-18)17-21-15(4,5)16(6,7)22-17/h8H,9-11H2,1-7H3
InChI 密鑰
VVDCRJGWILREQH-UHFFFAOYSA-N
一般說明
Boc-THP-Bpin is frequently used in metal catalyzed cross-coupling reactions, such as Suzuki-Miyaura and Negishi couplings.
應用
Reagent used for
Reagent used in Preparation of several enzymatic inhibitors and receptor ligands
- Suzuki-Miyaura cross-coupling using palladium phosphine catalyst
- Palladium-catalyzed ligand-controlled regioselective Suzuki coupling
- Palladium-catalyzed Suzuki-Miyaura coupling
- Suzuki coupling followed by iodolactonization reaction
- Wrenchnolol derivative optimized for gene activation in cells
Reagent used in Preparation of several enzymatic inhibitors and receptor ligands
- Orally active anaplastic lymphoma kinase inhibitors
- Oxazolecarboxamides as diacylglycerol acyltransferase-1 inhibitors for treatment of obesity and diabetes
- 4-arylpiperidinyl amides and N-arylpiperidin-3-yl-cyclopropanecarboxamides as novel melatonin receptor ligands
- Quinazoline analogs as glucocerebrosidase inhibitors with chaperone activity for treatment of Gaucher disease, a lysosomal storage disorder
- Arylpiperazine and piperidine ethers as dual acting norepinephrine reuptake inhibitors and 5-HT1A partial agonists
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
Kazutomo Kinoshita et al.
Bioorganic & medicinal chemistry, 20(3), 1271-1280 (2012-01-10)
Anaplastic lymphoma kinase (ALK) receptor tyrosine kinase is considered an attractive therapeutic target for human cancers, especially non-small cell lung cancer (NSCLC). Our previous study revealed that 8,9-side-chains of 6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole scaffold crucially affected kinase selectivity, cellular activity, and metabolic stability.
Preparation of 3,4-fused-spiro[furan-5(5H),4'-piperidin]-2-one
Liu, J.; et al.
Tetrahedron Letters, 50, 5228-5230 (2009)
Design and synthesis of 4-arylpiperidinyl amide and N-arylpiperidin-3-yl-cyclopropanecarboxamide derivatives as novel melatonin receptor ligands
Li, G.; et al.
Bioorganic & Medicinal Chemistry, 21, 1236-1242 (2011)
Paul M Wehn et al.
Organic letters, 11(24), 5666-5669 (2009-12-17)
A novel approach to the synthesis of substituted 5-amino- and 3-amino-1,2,4-thiadiazoles beginning from a common precursor has been achieved. Derivatization by palladium-catalyzed Suzuki-Miyaura coupling enables the rapid preparation of analogs around this pharmaceutically relevant core. FMO calculations rationalize the observed
Dongju Jung et al.
Journal of the American Chemical Society, 131(13), 4774-4782 (2009-03-18)
Naturally occurring transcription factors usually have two independent domains, a DNA-binding domain and an activation domain. In designing a synthetic small molecule that mimics a transcription factor, each of the two domains needs to be replaced by small-molecule counterparts. Results
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门





![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

