所有图片(3)
About This Item
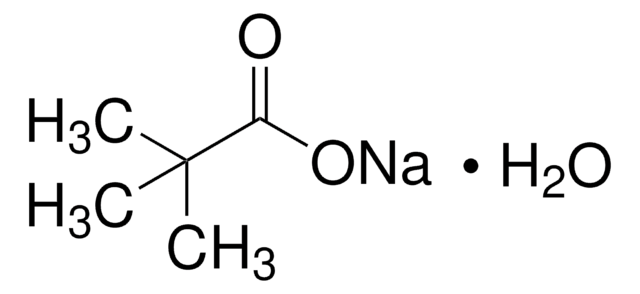
经验公式(希尔记法):
C5H9O2Cs
CAS号:
分子量:
234.03
MDL號碼:
分類程式碼代碼:
12352302
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
98%
形狀
powder
mp
344-348 °C
SMILES 字串
[Cs+].CC(C)(C)C([O-])=O
InChI
1S/C5H10O2.Cs/c1-5(2,3)4(6)7;/h1-3H3,(H,6,7);/q;+1/p-1
InChI 密鑰
LGVUAXNPXVXCCW-UHFFFAOYSA-M
一般說明
特戊酸铯是一种有机碱,由于其在有机溶剂中的可溶性,广泛用于钯催化的交叉耦合和羰基化反应。
應用
特戊酸铯可作为碱用于合成:
- 芴-9-酮衍生物,通过钯催化环羰基化 邻-卤代联芳基。
- 稠合杂环(二氢苯并呋喃和二氢吲哚)由 邻-溴苯酚和苯胺前体通过Pd催化两个C(sp3)-H键的分子内偶联得到。
- 含有季 β-碳原子的酰胺和酯类衍生物,通过Pd催化的C-H活化和氨基/烷氧基羰基化反应制备。
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
M A Campo et al.
Organic letters, 2(23), 3675-3677 (2000-11-14)
The synthesis of various substituted fluoren-9-ones has been accomplished by a novel palladium-catalyzed cyclocarbonylation of o-halobiaryls. The cyclocarbonylation of 4'-substituted-2-iodobiphenyls produces very high yields of 2-substituted fluoren-9-ones bearing either electron-donating or electron-withdrawing substituents. 3'-Substituted 2-iodobiphenyls afford in excellent yields with
Tomáš Čarný et al.
Angewandte Chemie (International ed. in English), 59(43), 18980-18984 (2020-07-22)
The 1,4-palladium shift strategy allows the functionalization of remote C-H bonds that are difficult to reach directly. Reported here is a domino reaction proceeding by C(sp3 )-H activation, 1,4-palladium shift, and amino- or alkoxycarbonylation, which generates a variety of amides
Ronan Rocaboy et al.
Angewandte Chemie (International ed. in English), 58(41), 14625-14628 (2019-08-10)
The intramolecular coupling of two C(sp3 )-H bonds to forge a C(sp3 )-C(sp3 ) bond is enabled by 1,4-Pd shift from a trisubstituted aryl bromide. Contrary to most C(sp3 )-C(sp3 ) cross-dehydrogenative couplings, this reaction operates under redox-neutral conditions, with
Zubaoyi Yi et al.
The Journal of organic chemistry, 82(13), 6946-6957 (2017-06-16)
Pd-catalyzed arylation or benzylation of nitroazoles using aryl sulfonates or benzyl acetates is described. Electronically varied aryl tosylates and mesylates, as well as benzyl acetates, afford the arylated and benzylated products. Arylation of nitrobenzene is also reported. The relative rate
Aditya L Gottumukkala et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 17(11), 3091-3095 (2011-02-10)
Ace of base: A catalytic system is presented that, solely by choice of the base, selectively switches between conjugate addition and the Mizoroki-Heck reaction of aryl halides with Michael acceptors (see scheme; R, R' = alkyl, aryl). For conjugate addition
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门


![1,8-二氮杂双环[5.4.0]十一碳-7-烯 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)