所有图片(2)
About This Item
经验公式(希尔记法):
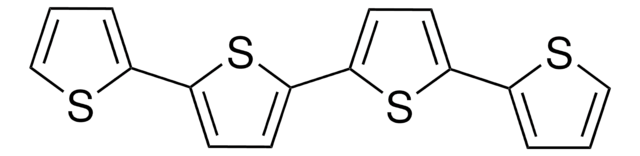
C12H8S3
CAS号:
分子量:
248.39
Beilstein:
178604
MDL编号:
UNSPSC代码:
12352103
PubChem化学物质编号:
NACRES:
NA.23
推荐产品
方案
99%
mp
93-95 °C (lit.)
SMILES字符串
c1csc(c1)-c2ccc(s2)-c3cccs3
InChI
1S/C12H8S3/c1-3-9(13-7-1)11-5-6-12(15-11)10-4-2-8-14-10/h1-8H
InChI key
KXSFECAJUBPPFE-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般描述
2,2′:5′,2′′-三噻吩(TTh)可通过衍生自2-溴噻吩的格氏试剂和镁的镍催化偶联反应而制备。它可产生单线态氧。在自然界中,存在于印加孔雀草和Echinops grijisii的花卉提取物中。已知它对蚊子有毒。它还具有抗真菌活性。
应用
咔唑和TTh在高氯酸钠/乙腈中的电化学共聚合已被报道。基于TTh和3,4-亚乙二氧基噻吩的电致变色共聚物已被报道。TTh可充当聚噻吩的单体前体以及聚碳酸酯的掺杂剂。它可用作光敏剂。
在四丁基高氯酸铵溶液中,3T可结合 3,4-乙烯二氧噻吩 (EDOT),作为电致变色共聚物用于各种应用,如光伏发电和聚合物发光二极管 (LED)。它也可以和铝、银和钙等金属一起形成金属有机物薄膜,用于光电子学应用等。
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
Eyeshields, Gloves, type N95 (US)
历史批次信息供参考:
分析证书(COA)
Lot/Batch Number
M J Perich et al.
Journal of the American Mosquito Control Association, 11(3), 307-310 (1995-09-01)
Application of Tagetes minuta floral extract to silica gel column chromatography produced 2 fractions with the hydrogenate part 20-30 times more toxic to larvae and 12-13 times more toxic to adults of Aedes aegypti and Anopheles stephensi, respectively, than the
Sanami Yazaki et al.
Journal of the American Chemical Society, 132(22), 7702-7708 (2010-05-15)
New molecular materials combining ionic and electronic functions have been prepared by using liquid crystals consisting of terthiophene-based mesogens and terminal imidazolium groups. These liquid crystals show thermotropic smectic A phases. Nanosegregation of the pi-conjugated mesogens and the ionic imidazolium
Generation of singlet oxygen by 2,2:5,2-terthiophene and some of its derivatives.
Ciofalo M, et al.
Journal of Photochemistry and Photobiology A: Chemistry, 83(1), 1-6 (1194)
Synthesis and characterization of an electrochromic copolymer based on 2,2':5',"-terthiophene and 3,4-ethylenedioxythiophene
Ahmed MS, et al.
Applied Nanoscience, 2, 133-141 (2012)
Bulletin of the Chemical Society of Japan, 66, 2960-2960 (1993)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持




![苯并[1,2B:4,5-B]二噻吩-4- -1,8-二酮 97%](/deepweb/assets/sigmaaldrich/product/structures/418/544/b7faac0b-ad09-4b42-a9fa-aeb38017a39e/640/b7faac0b-ad09-4b42-a9fa-aeb38017a39e.png)
