所有图片(3)
About This Item
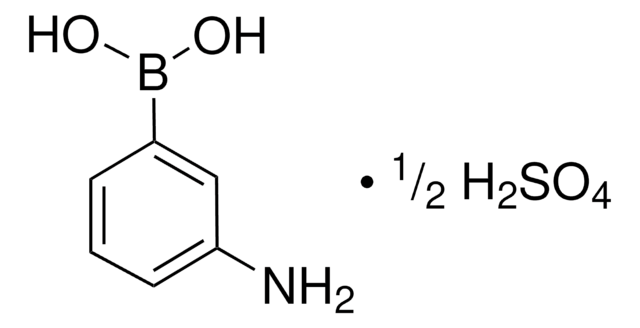
线性分子式:
H2NC6H4B(OH)2 · H2O
CAS号:
分子量:
154.96
Beilstein:
2936342
MDL號碼:
分類程式碼代碼:
12352103
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
98%
形狀
solid
mp
93-96 °C (lit.)
SMILES 字串
[H]O[H].Nc1cccc(c1)B(O)O
InChI
1S/C6H8BNO2.H2O/c8-6-3-1-2-5(4-6)7(9)10;/h1-4,9-10H,8H2;1H2
InChI 密鑰
XAEOVQODHLLNKX-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
用于
用于制备
- Suzuki-Miyaura交叉偶联的试剂
用于制备
- 无乳链球菌(Streptococcus agalactiae) STK1革兰氏阳性抗毒药物和抑制剂的试剂
- Zaleplon的区域异构体(镇静剂)
- 两亲性随机糖聚合物,其可自组装形成纳米颗粒,具有作为葡萄糖敏感基质的潜力
- 化学机械聚合物,可在血浆中膨胀和收缩,具有高葡萄糖选择性
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Mayalen Oxoby et al.
Bioorganic & medicinal chemistry letters, 20(12), 3486-3490 (2010-06-10)
A structure-activity relationship study from a screening hit and structure-based design strategy has led to the identification of bisarylureas as potent inhibitors of Streptococcus agalactiae Stk1. As this target has been directly linked to bacterial virulence, these inhibitors can be
A chemomechanical polymer that functions in blood plasma with high glucose selectivity.
George K Samoei et al.
Angewandte Chemie (International ed. in English), 45(32), 5319-5322 (2006-08-24)
Qianqian Guo et al.
Journal of biomaterials science. Polymer edition, 30(10), 815-831 (2019-05-03)
We reported on the fabrication of sugar-responsive nanogels covalently incorporated with 3-acrylamidophenylboronic acid (AAPBA) as glucose-recognizing moiety, 2-(acrylamido)glucopyranose (AGA) as biocompatible moiety, and boron dipyrromethene (BODIPYMA) as fluorescence donor molecule. The p(AAPBA-AGA-BODIPYMA) nanogels were synthesized via reversible addition-fragmentation chain transfer
Synthetic studies connected with the preparation of N-[3-(3-cyanopyrazolo[1,5-a]pyrimidin-5-yl)phenyl]-N-ethylacetamide, a zaleplon regioisomer
Radl, S.; et al.
Heterocycles, 80, 1359-1379 (2010)
Lujie Yang et al.
The Analyst, 145(15), 5252-5259 (2020-07-04)
Glycosylation is an important mechanism of secondary protein processing. Large-scale profiling of glycopeptides released by proteolytic digestion of glycoproteins from biologic samples with complex compositions is limited due to their low abundance. Herein, we present a multimodal material based on
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门