推荐产品
化驗
98%
形狀
powder
mp
76-79 °C (lit.)
SMILES 字串
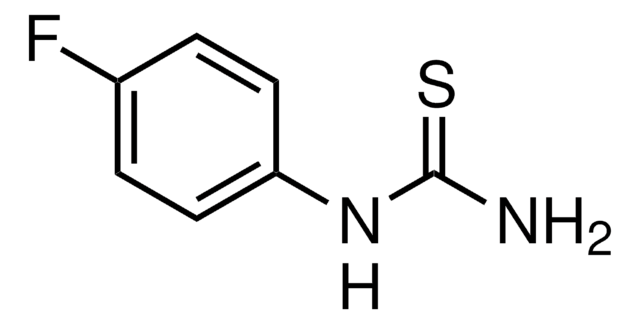
CC(=S)Nc1ccccc1
InChI
1S/C8H9NS/c1-7(10)9-8-5-3-2-4-6-8/h2-6H,1H3,(H,9,10)
InChI 密鑰
MWCGLTCRJJFXKR-UHFFFAOYSA-N
一般說明
Metabolism and acute toxicity of thioacetanilide has been studied in rat. Thioacetanilide undergoes nucleophilic addition reaction with superoxide ion in dimethyl sulfoxide.
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Anna Michta et al.
Acta crystallographica. Section C, Crystal structure communications, 64(Pt 8), o411-o413 (2008-08-07)
The title compound, C(8)H(9)NS, has four symmetry-independent molecules in the asymmetric unit. These molecules link into two independent infinite N-H...S hydrogen-bonded chains in the a-axis direction with graph-set notation C(2)(2)(8). The NH-CS group adopts a trans conformation and forms a
Peng Zhan et al.
Bioorganic & medicinal chemistry, 17(16), 5775-5781 (2009-08-01)
A series of 2-(1-aryl-1H-imidazol-2-ylthio)acetamide [imidazole thioacetanilide (ITA)] derivatives were synthesized and evaluated as potent inhibitors of human immunodeficiency virus type-1 (HIV-1). Among them, the most potent HIV-1 inhibitors were 4a5 (EC(50)=0.18microM), and 4a2 (EC(50)=0.20microM), which were more effective than the
[Substantiation of maximum permissible levels of thioacylanilide in the air of work areas].
L G Aĭzvert et al.
Gigiena truda i professional'nye zabolevaniia, (7)(7), 51-52 (1986-07-01)
Reactivity of superoxide ion with thioamides in dimethyl sulfoxide.
Paez OA, et al.
The Journal of Organic Chemistry, 53(10), 2166-2170 (1988)
Xiao Li et al.
Bioorganic & medicinal chemistry, 20(18), 5527-5536 (2012-08-14)
In continuation of our efforts toward the discovery of potent HIV-1 NNRTIs with novel structures, we have employed a scaffold hopping strategy to explore the chemically diversed space of bioactive compounds. The original arylazolylthioacetanilide platform was replaced with different imidazopyridinylthioacetanilide
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门