推荐产品
一般說明
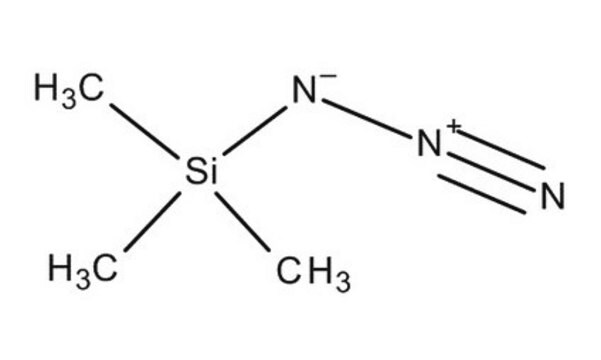
叠氮基三甲基硅烷(TMSN3)是一种无色稳定的有机硅烷试剂。其在高温下分解非常缓慢。 它是一种非常常用的叠氮化物来源,已用于合成氨基三唑配体。通过将氯三甲基硅烷滴加到搅拌的NaN3 二甘醇二甲醚溶液中,可以非常容易地合成叠氮基三甲基硅烷。
應用
叠氮三甲基硅烷可用作:
- 通过金属有机化学气相沉积方法制备 GaN 纳米线的氮前驱体。
- Li-O2 电池中的电解质添加剂。TMSN3 的加入可形成坚固的固体电解质界面。
- 这是合成四唑、富勒烯叠氮和 α-叠氮肟的有效试剂。
- 是醇和酚的 O-三甲基甲硅烷基化中的甲硅烷基化度试剂。
訊號詞
Danger
危險分類
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
42.8 °F - closed cup
閃點(°C)
6 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Azidotrimethylsilane
Li, B. L.
Synlett, 23(10), 1554-1555 (2012)
Synthesis, 106-106 (1988)
Covalent functionalization of epitaxial graphene by azidotrimethylsilane
Choi, Junghun, et al.
The Journal of Physical Chemistry C, 113(22), 9433-9435 (2009)
James T Goettel et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 26(5), 1136-1143 (2019-11-30)
A cyclic (alkyl)(amino)carbene (CAAC) has been shown to react with a covalent azide similar to the Staudinger reaction. The reaction of Me CAAC with trimethylsilyl azide afforded the N-silylated 2-iminopyrrolidine (Me CAAC=NSiMe3 ), which was fully characterized. This compound undergoes
Journal of the American Chemical Society, 115, 9347-9347 (1993)
商品
Click chemistry, and the copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) in particular, is a powerful new synthetic tool in polymer chemistry and material science.
Since the preparation of the first organic azide, phenyl azide, by Peter Griess in 1864 this energy-rich and versatile class of compounds has enjoyed considerable interest.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门