推荐产品
一般說明
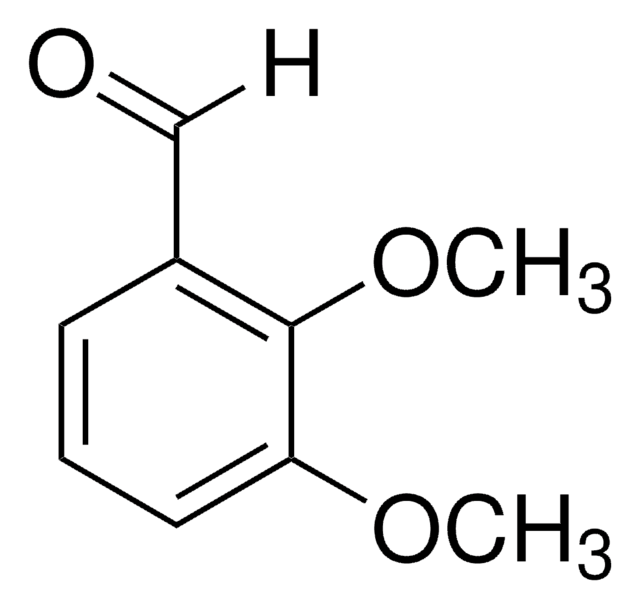
3,4-二甲氧基苯甲醛与环糊精形成1:1的包合物。它与3-乙酰基-2,5-二甲基噻吩反应生成查尔酮染料2E)-3-(3,4-二甲氧基苯基)-1-(2,5-二甲基噻吩-3-基)丙-2-烯-1-酮。
應用
3,4-二甲氧基苯甲醛用于制备4-氯甲基-2-(二甲氧基苯基)-1,3-二氧戊环。用于合成具有抗HIV活性的(+)-紫草酸。
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Skin Irrit. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
235.4 °F - closed cup
閃點(°C)
113 °C - closed cup
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
2-(Multimethoxy) phenyl-4-methylene-1, 3-dioxolane (I): Preparation and Cationic Polymerization of 2-(Dimethoxy) phenyl-4-MDO Derivatives.
Kim JT and Gong M-S.
Bull. Korean Chem. Soc., 20, 663-666 (1999)
Tirumala G Varadaraju et al.
Organic & biomolecular chemistry, 10(28), 5456-5465 (2012-06-07)
An efficient and convergent route for the synthesis of the natural product (+)-lithospermic acid, which possesses anti-HIV activity, was accomplished. The (±)-trans-dihydrobenzo[b]furan core therein was prepared by two different strategies. The first strategy involved the use of a palladium-catalyzed annulation
Abdullah M Asiri et al.
Journal of fluorescence, 23(6), 1271-1278 (2013-07-13)
This study introduced spectroscopic properties, physicochemical parameters, and polarity and photostability behaviors of a newly prepared chalcone dye. The chalcone dye, (2E)-3-(3,4-Dimethoxyphenyl)-1-(2,5-dimethylthiophen-3-yl)prop-2-en-1-one (DDTP), was synthesized by the reaction of 3,4-dimethoxybenzaldehyde with 3-acetyl-2,5-dimethythiophene. Results of FT-IR, (1)H-NMR, (13)C-NMR and elemental analysis
M Jude Jenita et al.
Journal of fluorescence, 24(3), 695-707 (2013-12-07)
The inclusion complexation of 2-hydroxy-3-methoxybenzaldehyde (2HMB), 4-hydroxy-3-methoxybenzaldehyde (4HMB), 3,4-dimethoxybenzaldehyde (DMB) and 4-hydroxy-3,5-dimethoxybenzaldehyde (HDMB) with α-CD, β-CD, HP-α-CD and HP-β-CD were carried out by UV-Visible, steady-state and time-resolved fluorescence and PM3 methods. All the benzaldehydes shows dual fluorescence in aqueous and
J Kavitha et al.
Journal of Asian natural products research, 2(2), 157-160 (2001-03-17)
N-[3-(3,4-Dimethoxyphenyl)propanoyl]pyrrole (1) has been synthesized in three steps starting from veratraldehyde (2) with an overall yield of 66%.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门