04473
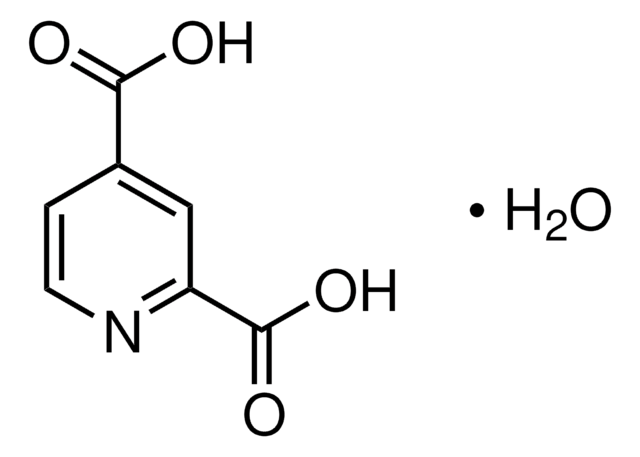
2,4-Pyridinedicarboxylic acid
≥98.0%
Synonyme(s) :
Lutidinic acid
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill):
C7H5NO4
Numéro CAS:
Poids moléculaire :
167.12
Numéro Beilstein :
131631
Numéro CE :
Numéro MDL:
Code UNSPSC :
12352106
ID de substance PubChem :
Nomenclature NACRES :
NA.25
Produits recommandés
Niveau de qualité
Pureté
≥98.0%
98.0-102.0% (T)
Chaîne SMILES
OC(=O)c1ccnc(c1)C(O)=O
InChI
1S/C7H5NO4/c9-6(10)4-1-2-8-5(3-4)7(11)12/h1-3H,(H,9,10)(H,11,12)
Clé InChI
MJIVRKPEXXHNJT-UHFFFAOYSA-N
Application
2,4-Pyridinedicarboxylic acid is an in vitro and in cell inhibitor, as well as a known inhibitor of the histone lysine demethylases. 2,4-Pyridinedicarboxylic acid has been used in a study to determine that ruthenium(II) complexes exert antimetastatic effects on several tumor cell lines in vitro, achieved mostly by the effect on cell adhesion, migration and angiogenesis. . 2,4-Pyridinedicarboxylic acid has been used in a study to develop an assay that represents the first report of a RapidFire mass spectrometery assay for an epigenetics target.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Michael Raghunath et al.
Biochemical and biophysical research communications, 378(4), 766-771 (2008-12-11)
The rapid vascularisation of biomaterials and engineered tissue after implantation is a current unmet need. To this end, we explored the pharmacological option of inducing neovascularisation using compounds that inhibit hypoxia-induced factor-1alpha prolyl hydroxylase. This stabilises hypoxia inducible factor-1alpha and
H M Rowe et al.
Applied spectroscopy, 57(5), 532-537 (2003-12-09)
An adaptation of square-wave gated phase-modulation (GPM) fluorimetry allows for self-referenced intensity measurements without the complexity of dual excitation or dual emission wavelengths. This AC technique utilizes square-wave excitation, gated detection, a reference emitter, and a sensor molecule. The theory
John R G Sander et al.
Journal of pharmaceutical sciences, 99(9), 3676-3683 (2010-06-25)
We report on a co-crystal of acetaminophen (APAP) and 2,4-pyridinedicarboxylic acid (PDA). The co-crystal was discovered by screening using the solution-mediated phase transformation (SMPT) technique. Despite the bulk solids of each component being white in color, the new co-crystal phase
C K Derian et al.
The Journal of biological chemistry, 264(12), 6615-6618 (1989-04-25)
While a role has been ascribed to the gamma-carboxyglutamate (Gla) residues in vitamin K-dependent coagulation proteins and the enzyme catalyzing this posttranslational modification has been identified and partially characterized, both the functional significance of a second posttranslationally synthesized amino acid
Guo-liang Gu et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 71(1), 209-214 (2008-02-19)
Two novel ligands containing two pyridine-2,6-dicarboxylic acid conjugative units, 4-(2-(2,6-dicarbox-ypyridin-4-yl)vinyl)pyridine-2,6-dicarboxylic acid (L1) and 4-(4-(2-(2,6-dicarboxypyridin-4-yl)vinyl)styryl)pyridine-2,6-dicarboxylic acid (L2) and their complexes with Tb(III) have been synthesized and characterized by elemental analysis, IR spectra and NMR. The ligand synthetic route was optimized and
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique