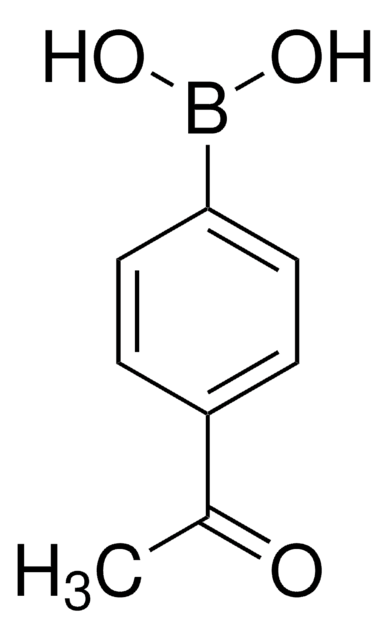
456772
4-Carboxyphenylboronic acid
Synonyme(s) :
4-(Dihydroxyboronyl)benzoic acid, 4-(Dihydroxyboryl)benzoic acid, 4-Boronobenzoic acid, 4-Carboxybenzeneboronic acid, 4-Carboxylphenylboronic acid, 4-Hydroxycarbonylphenyl boronic acid, NSC 221170, p-Boronobenzoic acid, p-Carboxybenzeneboronic acid, p-Carboxyphenylboronic acid
About This Item
Produits recommandés
Niveau de qualité
Pf
220 °C (dec.) (lit.)
Groupe fonctionnel
carboxylic acid
Chaîne SMILES
OB(O)c1ccc(cc1)C(O)=O
InChI
1S/C7H7BO4/c9-7(10)5-1-3-6(4-2-5)8(11)12/h1-4,11-12H,(H,9,10)
Clé InChI
SIAVMDKGVRXFAX-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Application
- Condensation reactions with stabilizer chains at the surface of polystyrene latex
- Suzuki coupling reactions
- Esterification
- Derivatization of polyvinylamine
- Synthesis of isotopically labeled mercury
- Functionalization of poly-SiNW for detection of dopamine
- Suzuki-Miyaura cross-coupling[1]
- Induction of pH sensitivity on fluorescence lifetime of quantum dots by NIR fluorescent dyes[2]
- Bio-supported palladium nanoparticles as phosphine-free catalyst for Suzuki reaction in water[3]
- Chan-Lam-type Copper (Cu)-catalyzed S-arylation with aryl boronic acids at room temperature[4]
Reagent used in Preparation of
- Isoquinolones via regioselective Suzuki-Miyaura cross-coupling and tandem palladium-catalyzed intramolecular aminocarbonylation and annulation[5]
- Amprenavir-based P1-substituted bi-aryl derivatives as ultra-potent HIV-1 protease inhibitors[6]
- Phenols via visible-light initiated aerobic oxidative hydroxylation of arylboronic acids using air as oxidant catalyzed by Ruthenium (Ru)-complex[7]
- Glucose sensitive boronic acid-bearing block copolymers[8]
- Trisulfonated calixarene upper-rim sulfonamido derivatives and their complexation with the trimethyllysine epigenetic mark[1]
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Vous ne trouvez pas la bonne version ?
Si vous avez besoin d'une version particulière, vous pouvez rechercher un certificat spécifique par le numéro de lot.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Active Filters
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique