763039
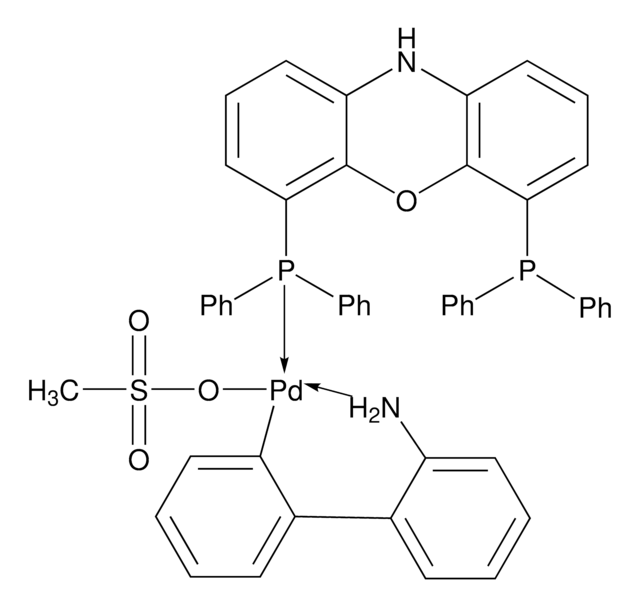
XantPhos Pd G3
95%
Sinónimos:
[(4,5-Bis(diphenylphosphino)-9,9-dimethylxanthene)-2-(2′-amino-1,1′-biphenyl)]palladium(II) methanesulfonate
About This Item
Productos recomendados
Nivel de calidad
Análisis
95%
formulario
solid
Características
generation 3
idoneidad de la reacción
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
reaction type: Cross Couplings
mp
164-167 °C (decomposition)
grupo funcional
phosphine
cadena SMILES
CS(=O)(=O)O[Pd]c1ccccc1-c2ccccc2N.CC3(C)c4cccc(P(c5ccccc5)c6ccccc6)c4Oc7c(cccc37)P(c8ccccc8)c9ccccc9
InChI
1S/C39H32OP2.C12H10N.CH4O3S.Pd/c1-39(2)33-25-15-27-35(41(29-17-7-3-8-18-29)30-19-9-4-10-20-30)37(33)40-38-34(39)26-16-28-36(38)42(31-21-11-5-12-22-31)32-23-13-6-14-24-32;13-12-9-5-4-8-11(12)10-6-2-1-3-7-10;1-5(2,3)4;/h3-28H,1-2H3;1-6,8-9H,13H2;1H3,(H,2,3,4);/q;;;+1/p-1
Clave InChI
AJVXPGQYAKUTGX-UHFFFAOYSA-M
Descripción general
Aplicación
- Negishi cross-coupling reaction during the synthesis of palmerolides.
- Aminocarbonylation of heteroaryl bromides with carbon monoxide (CO) in the presence of triethylamine.
- Coupling between polyglycosyl thiols and aglycon halides by C-S bond formation.
Producto relacionado
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Preformed catalysts in the kit are stable but become air-sensitive when activated; Schlenk technique aids scale-up.
All contents in the foil bag are weighed, plated, packed, and sealed in a glove box under nitrogen.
G3 and G4 Buchwald palladium precatalysts are the newest air, moisture, and thermally stable crossing-coupling complexes used in bond formation for their versatility and high reactivity.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico