C3743
CI 976
>98% (HPLC), solid
Synonym(e):
2,2-Dimethyl-N-(2,4,6-trimethoxyphenyl)dodecanamide
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
>98% (HPLC)
Form
solid
Löslichkeit
DMSO: >10 mg/mL
H2O: insoluble <2 mg/mL
Lagertemp.
2-8°C
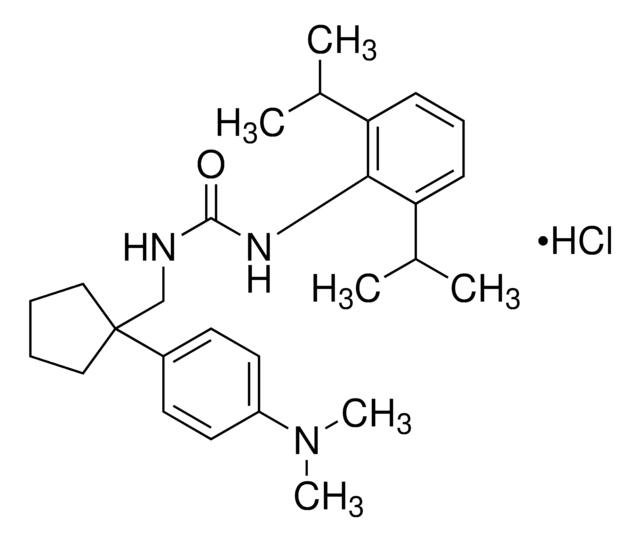
SMILES String
CCCCCCCCCCC(C)(C)C(=O)Nc1c(OC)cc(OC)cc1OC
InChI
1S/C23H39NO4/c1-7-8-9-10-11-12-13-14-15-23(2,3)22(25)24-21-19(27-5)16-18(26-4)17-20(21)28-6/h16-17H,7-15H2,1-6H3,(H,24,25)
InChIKey
WAFNZAURAWBNDZ-UHFFFAOYSA-N
Anwendung
- to analyze its anti-hepatitis C virus (HCV) activity in Huh7.5.1 cells
- to treat Neuro-2a cells to test its effect on plasma membrane integrated density of α4-SEPβ2 or α6-SEPβ2β3 nicotinic acetylcholine receptors (nAChRs)
- to study its effects on the anti-angiogenic activity of pyripyropenes in human umbilical vein endothelial cells
Biochem./physiol. Wirkung
H-Sätze
P-Sätze
Gefahreneinstufungen
Aquatic Chronic 4
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.