C3662
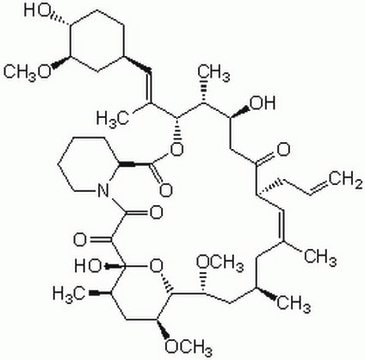
Cyclosporin A
from Tolypocladium inflatum, ≥95% (HPLC), solid, Calcineurin inhibitor
Synonyma:
Antibiotic S 7481F1, Cyclosporine
About This Item
Doporučené produkty
Název produktu
Cyclosporin A, from Tolypocladium inflatum, ≥95% (HPLC), solid
biological source
Tolypocladium inflatum
Quality Level
assay
≥95% (HPLC)
form
solid
color
white
solubility
dichloromethane: 10 mg/mL
ethanol: 10 mg/mL
DMSO: 50 mg/mL
chloroform: 6 mg/mL
H2O: insoluble
antibiotic activity spectrum
fungi
mode of action
enzyme | inhibits
originator
Novartis
storage temp.
2-8°C
SMILES string
CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C
InChI
1S/C62H111N11O12/c1-25-27-28-40(15)52(75)51-56(79)65-43(26-2)58(81)67(18)33-48(74)68(19)44(29-34(3)4)55(78)66-49(38(11)12)61(84)69(20)45(30-35(5)6)54(77)63-41(16)53(76)64-42(17)57(80)70(21)46(31-36(7)8)59(82)71(22)47(32-37(9)10)60(83)72(23)50(39(13)14)62(85)73(51)24/h25,27,34-47,49-52,75H,26,28-33H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b27-25+/t40-,41+,42-,43+,44+,45+,46+,47+,49+,50+,51+,52-/m1/s1
InChI key
PMATZTZNYRCHOR-CGLBZJNRSA-N
Gene Information
human ... PPIA(5478) , PPP3CA(5530) , PPP3CB(5532) , PPP3CC(5533) , PPP3R1(5534) , PPP3R2(5535)
Hledáte podobné produkty? Navštivte Průvodce porovnáváním produktů
Biochem/physiol Actions
Features and Benefits
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Carc. 1B - Repr. 1B
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
ppe
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Vyberte jednu z posledních verzí:
Osvědčení o analýze (COA)
Nevidíte správnou verzi?
Potřebujete-li konkrétní verzi, můžete vyhledat daný certifikát podle čísla dávky nebo čísla šarže.
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Zákazníci si také prohlíželi
Sortimentní položky
Bioactive small molecules for immune system signaling target identification/validation and antibiotics, antivirals, and antifungals offered.
Discover Bioactive Small Molecules for ADME/Tox
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.