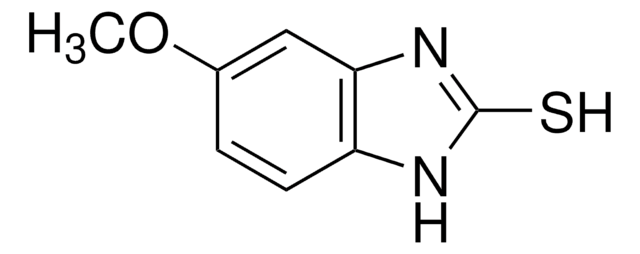
PHR1331
Metformin Related Compound A
Pharmaceutical Secondary Standard; Certified Reference Material
Synonyma:
Dicyandiamide, Cyanoguanidine, Dicyanodiamide
About This Item
Doporučené produkty
grade
certified reference material
pharmaceutical secondary standard
Quality Level
agency
traceable to USP 1396310
API family
metformin
CofA
current certificate can be downloaded
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
mp
208-211 °C (lit.)
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-8°C
SMILES string
NC(=N)NC#N
InChI
1S/C2H4N4/c3-1-6-2(4)5/h(H4,4,5,6)
InChI key
QGBSISYHAICWAH-UHFFFAOYSA-N
Hledáte podobné produkty? Navštivte Průvodce porovnáváním produktů
General description
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
It is an impurity of the widely used antidiabetic drug, metformin, that belongs to the biguanide class of compounds.
Application
- Separation and estimation of metformin along with other antidiabetics from meglitinides class in the presence of metformin impurity cyanoguanidine using a liquid chromatography method in pharmaceutical formulations
- Development of a reversed phase-high performance liquid chromatographic (RP-HPLC) method combined with a UV-diode array detector (PDA) for the impurity analysis of combined dosage tablet of metformin hydrochloride and teneligliptin hydrobromide hydrate
- Estimation of empagliflozin, linagliptin, along with metformin and its related impurities cyanoguanidine and melamine using HPLC-DAD and high-performance thin layer chromatography (HPTLC) methods in their combined tablets
- Development and validation of a stability indicating capillary zone electrophoresis (CZE) method for the quantification of metformin and its major impurity in pharmaceutical formulation
- Simultaneous determination of vildagliptin, metformin, and metformin-related compounds (A, B, and C) in tablets using a high-performance liquid chromatography-tandem mass spectrometry method (HPLC-MS/MS)
Analysis Note
Footnote
related product
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Vyberte jednu z posledních verzí:
Osvědčení o analýze (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Dokumenty section.
Potřebujete-li pomoc, obraťte se na Zákaznická podpora
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Zákazníci si také prohlíželi
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.