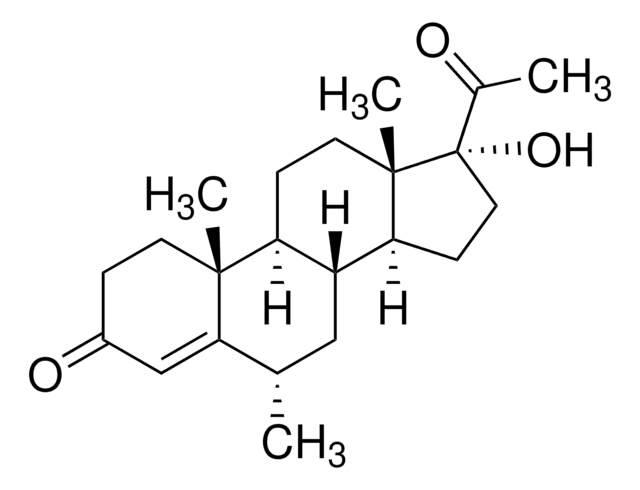
M0250000
Medroxyprogesterone acetate
European Pharmacopoeia (EP) Reference Standard
Synonyma:
Medroxyprogesterone 17-acetate, 17α-Acetoxy-6α-methylprogesterone, 17α-Hydroxy-6α-methyl-4-pregnene-3,20-dione 17-acetate, 6α-Methyl-17α-acetoxyprogesterone, 6α-Methyl-17α-hydroxyprogesterone acetate
About This Item
Doporučené produkty
grade
pharmaceutical primary standard
API family
medroxyprogesterone
manufacturer/tradename
EDQM
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
mp
206-207 °C (lit.)
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-8°C
SMILES string
[H][C@@]12C[C@H](C)C3=CC(=O)CC[C@]3(C)[C@@]1([H])CC[C@@]4(C)[C@@]2([H])CC[C@]4(OC(C)=O)C(C)=O
InChI
1S/C24H34O4/c1-14-12-18-19(22(4)9-6-17(27)13-21(14)22)7-10-23(5)20(18)8-11-24(23,15(2)25)28-16(3)26/h13-14,18-20H,6-12H2,1-5H3/t14-,18+,19-,20-,22+,23-,24-/m0/s1
InChI key
PSGAAPLEWMOORI-PEINSRQWSA-N
Gene Information
human ... PGR(5241)
Hledáte podobné produkty? Navštivte Průvodce porovnáváním produktů
General description
Application
Packaging
Other Notes
related product
signalword
Warning
hcodes
Hazard Classifications
Aquatic Chronic 4 - Carc. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
Vyberte jednu z posledních verzí:
Osvědčení o analýze (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Dokumenty section.
Potřebujete-li pomoc, obraťte se na Zákaznická podpora
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Zákazníci si také prohlíželi
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.