08198
Eleutheroside E
analytical standard
Synonyma:
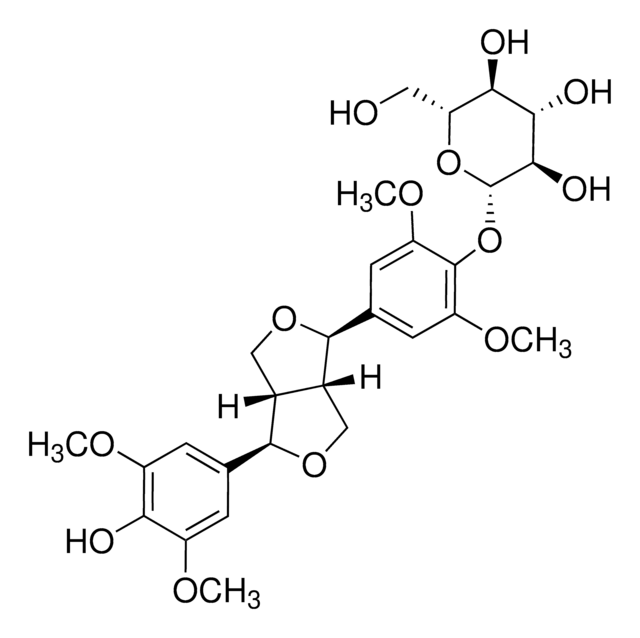
[(1R,3aR,4S,6aS)-Tetrahydro-1H,3H-furo[3,4-c]furan-1,4-diyl]bis(2,6-dimethoxy-4,1-phenylene) bis-β-D-glucopyranoside
About This Item
Doporučené produkty
grade
analytical standard
Quality Level
assay
≥98.0% (HPLC)
shelf life
limited shelf life, expiry date on the label
application(s)
food and beverages
format
neat
storage temp.
2-8°C
SMILES string
COc1cc(cc(OC)c1O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)[C@H]3OC[C@@H]4[C@@H]3CO[C@H]4c5cc(OC)c(O[C@@H]6O[C@H](CO)[C@@H](O)[C@H](O)[C@H]6O)c(OC)c5
InChI
1S/C34H46O18/c1-43-17-5-13(6-18(44-2)31(17)51-33-27(41)25(39)23(37)21(9-35)49-33)29-15-11-48-30(16(15)12-47-29)14-7-19(45-3)32(20(8-14)46-4)52-34-28(42)26(40)24(38)22(10-36)50-34/h5-8,15-16,21-30,33-42H,9-12H2,1-4H3/t15?,16?,21-,22+,23-,24+,25+,26-,27-,28+,29?,30?,33+,34-
InChI key
FFDULTAFAQRACT-RGFZIUCCSA-N
Hledáte podobné produkty? Navštivte Průvodce porovnáváním produktů
General description
Application
- Rat plasma and tissue by solid-phase extraction (SPE) followed by high-performance liquid chromatography (HPLC) and photodiode array detection (PDA).
- Acanthopanax senticosus by ionic liquids-ultrasound assisted extraction (ILUAE) followed by HPLC with ultraviolet (UV) detection.
- Eleutherococcus senticosus Maxim. by rapid resolution liquid chromatography (RRLC) equipped with multi-wavelength UV detector.
- Acanthopanax giraldii Harms by HPLC with diode array detector (DAD).
Packaging
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Vyberte jednu z posledních verzí:
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.