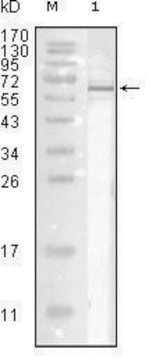
AB1074
Anti-Influenza A Antibody
Chemicon®, from goat
Přihlásitk zobrazení cen stanovených pro organizaci a smluvních cen
About This Item
UNSPSC Code:
12352203
eCl@ss:
32160702
NACRES:
NA.41
Doporučené produkty
biological source
goat
Quality Level
antibody form
purified immunoglobulin
clone
polyclonal
species reactivity
human
manufacturer/tradename
Chemicon®
technique(s)
ELISA: suitable
immunofluorescence: suitable
immunohistochemistry: suitable
western blot: suitable
shipped in
wet ice
Specificity
Specific for Influenza A by IHA. Recognizes H1N1 and H3N2 and probably other Flu A strains. Non reactive with HEp-2 cells. May react with chicken cellular proteins. Does not react with Influenza B, RSV, Parainfluenza 1/2/3 or Adenovirus.
Immunogen
Influenza A-USSR (H1N1).
Application
Anti-Influenza A Antibody is an antibody against Influenza A for use in ELISA, IF, IH & WB.
Applications include ELISA, fluorescence microscopy, immunoblotting; unboiled samples in 1mM DTT and 2% SDS only and immunohistochemistry. Final working dilutions must be determined by end user.
Titer: 1:1,000 by indirect immunofluorescence. (1:2,500 by hemagglutination inhibition.)
Titer: 1:1,000 by indirect immunofluorescence. (1:2,500 by hemagglutination inhibition.)
Research Category
Infectious Diseases
Infectious Diseases
Research Sub Category
Infectious Diseases - Viral
Infectious Diseases - Viral
Physical form
Format: Purified
Protein A Purified goat immunoglobulin in PBS (0.01 M, pH 7.2) with 0.1% sodium azide as a preservative.
Protein A purified
Storage and Stability
Maintain at +2–8°C for 3 months or at -20°C in aliquots for up to 12 months after date of receipt. Avoid repeated freeze/thaw cycles.
Analysis Note
Control
Influenza Control Slides, Catalogue Number 5010-5
Influenza Control Slides, Catalogue Number 5010-5
Other Notes
Concentration: Please refer to the Certificate of Analysis for the lot-specific concentration.
Legal Information
CHEMICON is a registered trademark of Merck KGaA, Darmstadt, Germany
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
recommended
Storage Class
10 - Combustible liquids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
Osvědčení o analýze (COA)
Vyhledejte osvědčení Osvědčení o analýze (COA) zadáním čísla šarže/dávky těchto produktů. Čísla šarže a dávky lze nalézt na štítku produktu za slovy „Lot“ nebo „Batch“.
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Zákazníci si také prohlíželi
Hao Chen et al.
Virology journal, 14(1), 242-242 (2017-12-24)
Numerous toxicological studies have focused on injury caused by exposure to single types of nanoparticles, but few have investigated how such exposures impact a host's immune response to pathogen challenge. Few studies have shown that nanoparticles can alter a host's
Wataru Yamazaki et al.
Transboundary and emerging diseases, 66(1), 341-348 (2018-09-30)
Transboundary animal diseases, including highly pathogenic avian influenza, cause vast economic losses throughout the world. While it is important to identify the sources and propagation routes of the spread, such strategies are often hindered by incomplete epidemiological evidence. Isolation/detection of
Amy Y Chang et al.
Respiratory research, 17(1), 62-62 (2016-05-25)
The hexapeptide SLIGRL-amide activates protease-activated receptor-2 (PAR-2) and mas-related G protein-coupled receptor C11 (MRGPRC11), both of which are known to be expressed on populations of sensory nerves. SLIGRL-amide has recently been reported to inhibit influenza A (IAV) infection in mice
Chih-Heng Huang et al.
The Journal of infectious diseases, 208(11), 1898-1905 (2013-08-01)
Reassortment within polymerase genes causes changes in the pathogenicity of influenza A viruses. We previously reported that the 2009 pH1N1 PA enhanced the pathogenicity of seasonal H1N1. We examined the effects of the PA gene from the HPAI H5N1 following
Mari Numata et al.
The Journal of biological chemistry, 295(6), 1704-1715 (2019-12-29)
The influenza A (H1N1)pdm09 outbreak in 2009 exemplified the problems accompanying the emergence of novel influenza A virus (IAV) strains and their unanticipated virulence in populations with no pre-existing immunity. Neuraminidase inhibitors (NAIs) are currently the drugs of choice for
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.