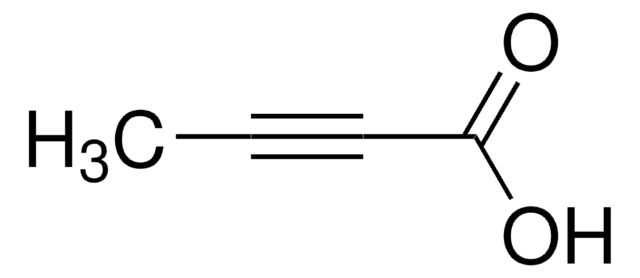P31205
Phenylpropiolic acid
99%
Synonyma:
Phenylpropynoic acid
Přihlásitk zobrazení cen stanovených pro organizaci a smluvních cen
About This Item
Lineární vzorec:
C6H5C≡CCOOH
Číslo CAS:
Molekulová hmotnost:
146.14
Beilstein/REAXYS Number:
742587
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Doporučené produkty
Quality Level
assay
99%
form
crystals
mp
135-137 °C (lit.)
storage temp.
2-8°C
SMILES string
OC(=O)C#Cc1ccccc1
InChI
1S/C9H6O2/c10-9(11)7-6-8-4-2-1-3-5-8/h1-5H,(H,10,11)
InChI key
XNERWVPQCYSMLC-UHFFFAOYSA-N
Hledáte podobné produkty? Navštivte Průvodce porovnáváním produktů
Application
Phenylpropiolic acid can:
- React with 2-tert-butoxypyridine in the presence of boron trifluoride·diethyl etherate to form the corresponding tert-butyl ester.
- Undergo decarboxylative coupling with aryl halides such as p-chloroiodobenzene and 1-chloro-4-iodobenzene.
- Undergo halodecarboxylation to form 1-haloalkynes.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Vyberte jednu z posledních verzí:
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Zákazníci si také prohlíželi
Rocío Gómez-Vásquez et al.
Annals of botany, 94(1), 87-97 (2004-05-18)
Control of diseases in the key tropical staple, cassava, is dependent on resistant genotypes, but the innate mechanisms are unknown. The aim was to study phenylpropanoids and associated enzymes as possible defence components. Phenylalanine ammonia-lyase (PAL), phenylpropanoids and peroxidases (POD)
Neil R McIntyre et al.
Journal of enzyme inhibition and medicinal chemistry, 31(4), 551-562 (2015-05-30)
Peptidylglycine α-amidating monooxygenase (PAM) is a bifunctional enzyme that catalyzes the final reaction in the maturation of α-amidated peptide hormones. Peptidylglycine α-hydroxylating monooxygenase (PHM) is the PAM domain responsible for the copper-, ascorbate- and O2-dependent hydroxylation of a glycine-extended peptide.
M Zieliński et al.
Isotopes in environmental and health studies, 37(3), 239-252 (2002-04-02)
13C kinetic isotope effect (KIE) in the decarboxylation of phenylpropiolic acid (PPA) in tetralin medium (Tn) has been determined at 409-432 K and found to be of magnitude similar to the 13C KIE observed in the decarboxylation of malonic acid
A fast and practical synthesis of tert-butyl esters from 2-tert-butoxypyridine using boron trifluoride? diethyl etherate under mild conditions.
La M T and Kim H K
Tetrahedron, 74(27), 3748-3754 (2018)
Elizabeth Joubert et al.
Food chemistry, 136(2), 1078-1085 (2012-11-06)
Z-2-(β-d-glucopyranosyloxy)-3-phenylpropenoic acid (PPAG), a compound postulated to contribute to the taste and mouthfeel of fermented rooibos tea (Aspalathus linearis), was isolated from unfermented rooibos plant material. Its structure was unequivocally confirmed by LC-MS, -MS(2), FT-IR and NMR of the underivatised
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.