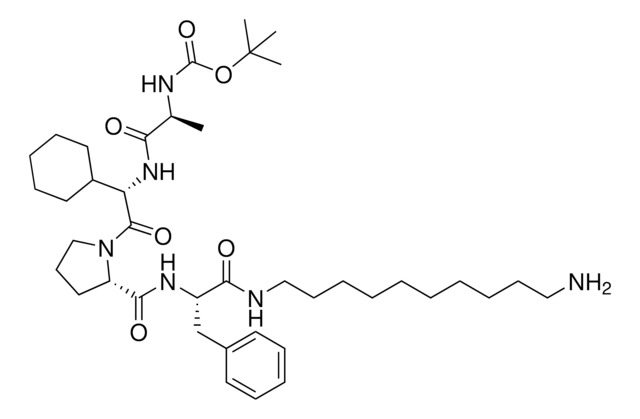
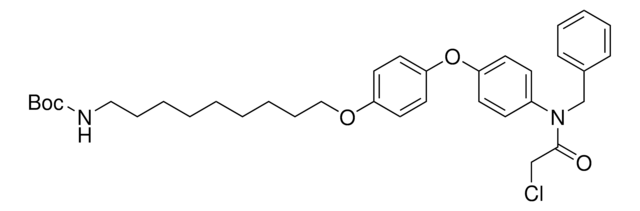
919489
CCW16-C6-PEG3-butyl-BocNH
Synonyma:
tert-butyl (6-(2-(2-((6-(4-(4-(N-Benzyl-2-chloroacetamido)phenoxy)phenoxy)hexyl)oxy)ethoxy)ethoxy)hexyl)carbamate, Crosslinker-E3 Ligase ligand conjugate, Protein degrader building block for PROTAC® research, RNF4-targeting building block, Template for synthesis of targeted protein degrader
About This Item
Doporučené produkty
ligand
CCW16
Quality Level
form
viscous liquid
reaction suitability
reactivity: carboxyl reactive
reagent type: ligand-linker conjugate
functional group
amine
storage temp.
2-8°C
SMILES string
O=C(CCl)N(CC1=CC=CC=C1)C2=CC=C(C=C2)OC3=CC=C(OCCCCCCOCCOCCOCCCCCCNC(OC(C)(C)C)=O)C=C3
Související kategorie
Application
Other Notes
Portal: Building PROTAC® Degraders for Targeted Protein Degradation
Covalent Ligand Screening Uncovers a RNF4 E3 Ligase Recruiter for Targeted Protein Degradation Applications
Targeted Protein Degradation by Small Molecules
Small-Molecule PROTACS: New Approaches to Protein Degradation
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
Legal Information
Related product
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Osvědčení o analýze (COA)
Vyhledejte osvědčení Osvědčení o analýze (COA) zadáním čísla šarže/dávky těchto produktů. Čísla šarže a dávky lze nalézt na štítku produktu za slovy „Lot“ nebo „Batch“.
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Sortimentní položky
Protein Degrader Building Blocks are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.


![Chloro[tris(para-trifluoromethylphenyl)phosphine]gold(I) 99%](/deepweb/assets/sigmaaldrich/product/structures/250/453/f96e05ee-0d9c-46a0-b0f5-818f89e15a2e/640/f96e05ee-0d9c-46a0-b0f5-818f89e15a2e.png)