902586
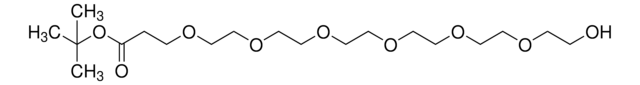
Hydroxy-PEG4-t-butyl ester
Synonyma:
tert-Butyl-1-hydroxy-3,6,9,12-tetraoxapentadecan-15-oate, HO-PEG4-CO-OtBu
Přihlásitk zobrazení cen stanovených pro organizaci a smluvních cen
About This Item
Empirický vzorec (Hillův zápis):
C15H30O7
Číslo CAS:
Molekulová hmotnost:
322.39
MDL number:
UNSPSC Code:
51171641
NACRES:
NA.22
Doporučené produkty
assay
≥95%
form
liquid
reaction suitability
reagent type: cross-linking reagent
refractive index
n/D 1.4492
density
1.04746 g/mL
functional group
ester
hydroxyl
storage temp.
2-8°C
SMILES string
O=C(OC(C)(C)C)CCOCCOCCOCCOCCO
InChI
1S/C15H30O7/c1-15(2,3)22-14(17)4-6-18-8-10-20-12-13-21-11-9-19-7-5-16/h16H,4-13H2,1-3H3
InChI key
FJRDXEGYAVAMLB-UHFFFAOYSA-N
Související kategorie
Application
This heterobifunctional, PEGylated crosslinker features a hydroxyl group at one end and t-butyl-protected carboxylic acid at the other, which can be deprotected with acidic conditions. The hydrophillic PEG linker facilitates solubility in biological applications. Hydroxy-PEG4-t-butyl ester can be used for bioconjugation or as a building block for synthesis of small molecules, conjugates of small molecules and/or biomolecules, or other tool compounds for chemical biology and medicinal chemistry that require ligation. Examples of applications include its synthetic incorporation into antibody-drug conjugates or proteolysis-targeting chimeras (PROTAC® molecules) for targeted protein degradation.
Other Notes
Legal Information
PROTAC is a registered trademark of Arvinas Operations, Inc., and is used under license
related product
Č. produktu
Popis
Stanovení ceny
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Vyberte jednu z posledních verzí:
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
A new route for the synthesis of 1-amino-3,6,9,12-?tetraoxapentadecan-15-oic acid.
Wu X, et al.
J. Chem. Res. (M), 40(6), 368-370 (2016)
Proline-functionalized magnetic core-shell nanoparticles as efficient and recyclable organocatalysts for aldol reactions.
Yacob Z, et al
Advanced Synthesis & Catalysis, 354(17), 3259-3264 (2012)
Synthesis of novel heterobifunctional isocyanato cross-linkers and their applications for the preparation of 10-hydroxycamptothecin and SN-38 conjugates with melanotransferrin P97.
Li Z, et al.
Synthetic Communications, 37(11) (2007)
Syntheses and characterizations of novel pyrrolocoumarin probes for SNAP-tag labeling technology.
Mei D, et al
Tetrahedron, 67(12), 2251-2259 (2011)
Recruiting cytotoxic T cells to folate-receptor-positive cancer cells.
Sumith A Kularatne et al.
Angewandte Chemie (International ed. in English), 52(46), 12101-12104 (2014-02-28)
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.