459941
(R)-(−)-Benzoin
98%
Synonyma:
(R)-2-Hydroxy-2-phenylacetophenone
Přihlásitk zobrazení cen stanovených pro organizaci a smluvních cen
About This Item
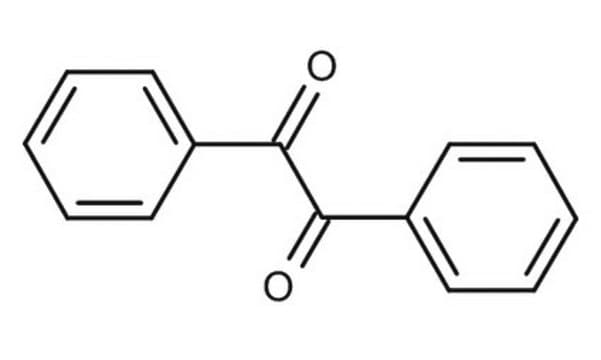
Lineární vzorec:
C6H5CH(OH)COC6H5
Číslo CAS:
Molekulová hmotnost:
212.24
MDL number:
UNSPSC Code:
12352115
PubChem Substance ID:
Doporučené produkty
assay
98%
optical activity
[α]24/D −115°, c = 1.5 in acetone
optical purity
ee: 99% (HPLC)
mp
135-137 °C (lit.)
functional group
hydroxyl
ketone
phenyl
SMILES string
O[C@H](c1ccccc1)C(=O)c2ccccc2
InChI
1S/C14H12O2/c15-13(11-7-3-1-4-8-11)14(16)12-9-5-2-6-10-12/h1-10,13,15H/t13-/m1/s1
InChI key
ISAOCJYIOMOJEB-CYBMUJFWSA-N
Application
(R)-(-)-Benzoin may be used in the preparation of (R)-2-hydroxy-1-phenylpropanone by reacting with benzaldehyde lyase (BAL) in the presence of acetaldehyde.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Vyberte jednu z posledních verzí:
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Hung-Wei Tsui et al.
Journal of chromatography. A, 1637, 461796-461796 (2021-01-03)
The effect of solvents on the enantioselectivities of four structurally similar chiral solutes with a cellulose derivative-based chiral stationary phase, Chiralpak IB, were studied using acetone (AC), 2-propanol (IPA), and tert-butanol (TBA) separately as polar modifiers. The enantioselectivities α of
Rüdiger Ohs et al.
Biotechnology progress, 35(6), e2868-e2868 (2019-06-18)
The kinetic description of enzyme-catalyzed reactions is a core task in biotechnology and biochemical engineering. In particular, mechanistic kinetic models help from the discovery of the biocatalyst throughout its application. Chemo- or enantioselective enzyme reactions often undergo two alternative pathways
Rüdiger Ohs et al.
Biotechnology progress, 34(5), 1081-1092 (2018-06-10)
Thiamine diphosphate (ThDP)-dependent enzymes catalyze a broad range of reactions with excellent enantioselectivity. Among these reactions, carboligations of aldehydes are of particular interest since the products, chiral hydroxy ketones, are valuable building blocks in the pharmaceutical industry. However, the substrates
Chromatograms
application for HPLCapplication for HPLCNáš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.