280348
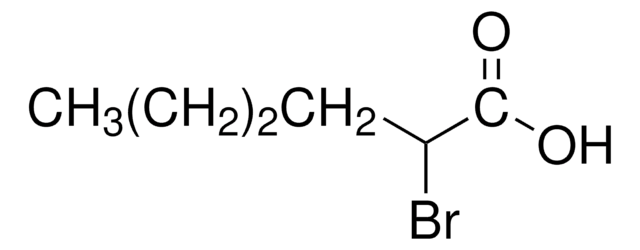
2-Bromooctanoic acid
97%
Synonyma:
2-Bromocaprylic acid
About This Item
Doporučené produkty
Quality Level
assay
97%
contains
Silver Wool as stabilizer
refractive index
n20/D 1.471 (lit.)
bp
140 °C/5 mmHg (lit.)
density
1.278 g/mL at 25 °C (lit.)
functional group
bromo
carboxylic acid
SMILES string
CCCCCCC(Br)C(O)=O
InChI
1S/C8H15BrO2/c1-2-3-4-5-6-7(9)8(10)11/h7H,2-6H2,1H3,(H,10,11)
InChI key
GTGTXZRPJHDASG-UHFFFAOYSA-N
Hledáte podobné produkty? Navštivte Průvodce porovnáváním produktů
General description
signalword
Danger
hcodes
Hazard Classifications
Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Vyberte jednu z posledních verzí:
Osvědčení o analýze (COA)
Nevidíte správnou verzi?
Potřebujete-li konkrétní verzi, můžete vyhledat daný certifikát podle čísla dávky nebo čísla šarže.
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Chromatograms
suitable for GCNáš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.