1.09864
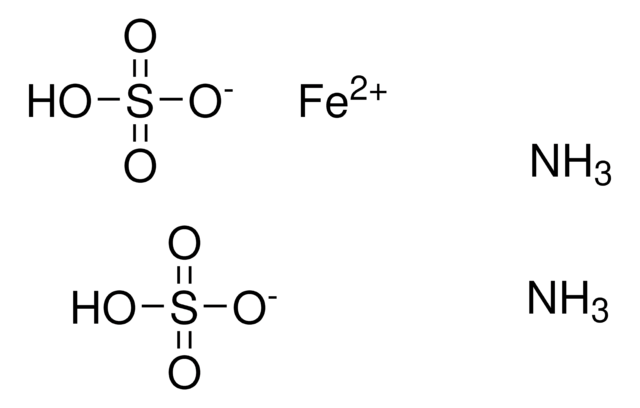
Ammonium iron(II) sulfate solution
c[(NH₄)₂ Fe(SO₄)₂] = 0.1 mol/l (0.1 N), concentrate for 250 mL titration solution, Titrisol®
Sinónimos:
Ammonium Ferrous Sulfate Solution
About This Item
Productos recomendados
product name
Ammonium iron(II) sulfate solution, for 250 mlc[(NH₄)₂ Fe(SO₄)₂] = 0.1 mol/l (0.1 N) Titrisol®
Quality Level
product line
Titrisol®
form
liquid
reaction suitability
reaction type: Complexometric reactions
concentration
0.1 M
technique(s)
titration: suitable
pH
0.4 (20 °C in H2O)
density
1.25 g/cm3 at 20 °C
storage temp.
15-25°C
Application
- Regulation of Iron-Ion Transporter SLC11A2 by Three Identical miRNAs.: This study investigates the modulation of iron ion transporters, providing insights into molecular processes involving ammonium iron(II) sulfate, crucial for analytical chemistry applications (Sugino et al., 2022).
- Two-Dimensional Bis(dithiolene)iron(II) Self-Powered UV Photodetectors with Ultrahigh Air Stability.: This research highlights the development of robust UV photodetectors using iron(II)-based materials, demonstrating the potential of ammonium iron(II) sulfate in creating high-performance electronic devices (Wang YC et al., 2021).
- Pencil it in: pencil drawn electrochemical sensing platforms.: Focuses on innovative, cost-effective electrochemical sensors where iron plays a critical role, pertinent to applications in chemical analysis (Foster CW et al., 2016).
- Catalytic effect of different forms of iron in purification of single-walled carbon nanotubes.: Discusses the use of various iron forms, including ammonium iron(II) sulfate, in enhancing the purification processes for carbon-based materials, significant in analytical applications (Suzuki T et al., 2010).
Legal Information
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Met. Corr. 1 - Skin Corr. 1A
Storage Class
8B - Non-combustible, corrosive hazardous materials
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico