ALD00564
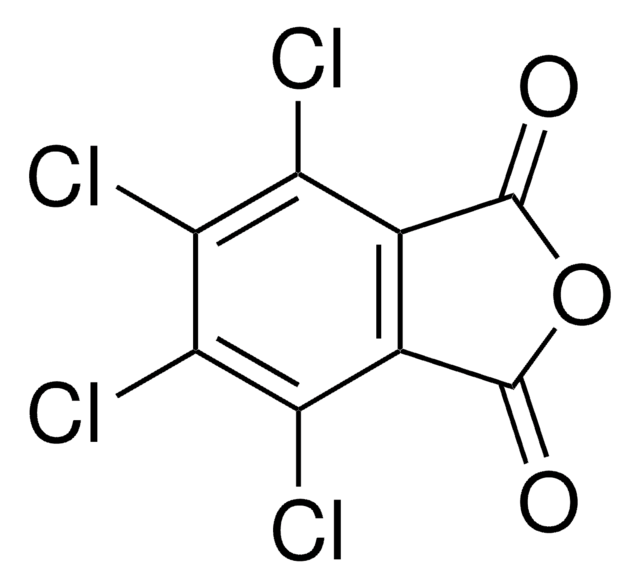
N-Hydroxytetrachlorophthalimide
Sinónimos:
4,5,6,7-Tetrachloro-2-hydroxy-1H-isoindole-1,3(2H)-dione, Tetrachloro-N-hydroxyphthalimide
About This Item
Productos recomendados
form
powder
Quality Level
reaction suitability
reagent type: oxidant
SMILES string
O=C1N(O)C(C2=C(Cl)C(Cl)=C(Cl)C(Cl)=C21)=O
InChI
1S/C8HCl4NO3/c9-3-1-2(4(10)6(12)5(3)11)8(15)13(16)7(1)14/h16H
InChI key
UTRBHXSKVVPTLY-UHFFFAOYSA-N
General description
Application
Other Notes
Scalable and sustainable electrochemical allylic C–H oxidation
A general alkyl-alkyl cross-coupling enabled by redox-active esters and alkylzinc reagents
Nickel-Catalyzed Cross-Coupling of Redox-Active Esters with Boronic Acids
Practical Ni-Catalyzed Aryl-Alkyl Cross-Coupling of Secondary Redox-Active Esters
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Oral
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Phil Baran develops ALD00564 reagent as a safe, cost-effective alternative for allylic oxidations and cross-coupling reactions.
Phil Baran develops ALD00564 reagent as a safe, cost-effective alternative for allylic oxidations and cross-coupling reactions.
Phil Baran develops ALD00564 reagent as a safe, cost-effective alternative for allylic oxidations and cross-coupling reactions.
Phil Baran develops ALD00564 reagent as a safe, cost-effective alternative for allylic oxidations and cross-coupling reactions.
Contenido relacionado
The Baran Group works with Sigma-Aldrich in providing a portfolio of zinc-based reagents promoting difluoromethylation, trifluoromethylation, trifluoroethylation and isopropylation of aryl and heteroaryl motifs. Baran’s lab has also helped introduce a portable desaturase (Tz0Cl), which promotes the installation of alcohol and amine groups and leaves behind a highly useful tosyl group for further transformations.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico