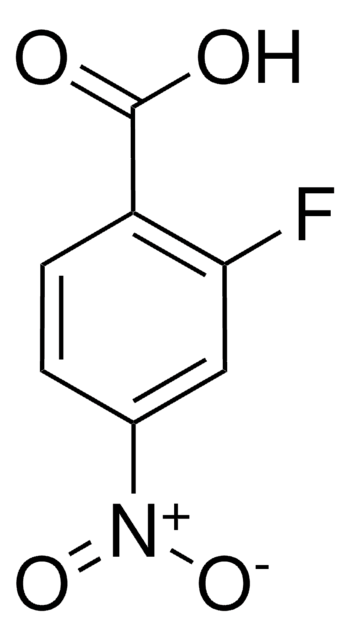
362654
2-Fluoro-5-nitrobenzoic acid
98%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
FC6H3(NO2)CO2H
Número de CAS:
Peso molecular:
185.11
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
98%
form
solid
mp
142-144 °C (lit.)
functional group
carboxylic acid
fluoro
nitro
SMILES string
OC(=O)c1cc(ccc1F)[N+]([O-])=O
InChI
1S/C7H4FNO4/c8-6-2-1-4(9(12)13)3-5(6)7(10)11/h1-3H,(H,10,11)
InChI key
ICXSHFWYCHJILC-UHFFFAOYSA-N
Categorías relacionadas
General description
2-Fluoro-5-nitrobenzoic acid is reported to react with an aldehyde, isonitrile and a primary amine tethered to a Boc-protected internal amino or hydroxyl nucleophile, to afford the Ugi product.
Application
2-Fluoro-5-nitrobenzoic acid may be used in the synthesis of:
- dibenz[b,f]oxazepin-11(10H)-ones, via the nucleophilic aromatic substitution (SNAr) of fluorine in 2-fluoro-5-nitrobenzoic acid with the OH of various 2-aminophenols on solid support
- synthesis of substituted dibenzazocines on solid support, via SNAr of fluorine in 2-fluoro-5-nitrobenzoic acid with the OH function of the immobilized polysubstituted phenols
- in the synthesis of oxazepines
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Solid support synthesis of 2-substituted dibenz [b, f] oxazepin-11 (10H)-ones< i> via</i> S< sub> N</sub> Ar methodology on AMEBA resin.
Ouyang X, et al.
Tetrahedron, 55(10), 2827-2834 (1999)
Efficient approach for the diversity-oriented synthesis of macro-heterocycles on solid-support.
Giulianotti M and Nefzi A.
Tetrahedron Letters, 44(28), 5307-5309 (2003)
Wim Meutermans et al.
ChemMedChem, 1(11), 1164-1194 (2006-09-20)
Drug discovery has long suffered from the difficulty of having to place pharmacophoric groups in just the right spatial arrangement to elicit the desired biological response. Although some molecule classes have been discovered that seem to be privileged structures for
MCC/SN Ar methodology. Part 2: Novel three-step solution phase access to libraries of benzodiazepines.
Tempest P, et al.
Tetrahedron Letters, 44(9), 1947-1950 (2003)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico