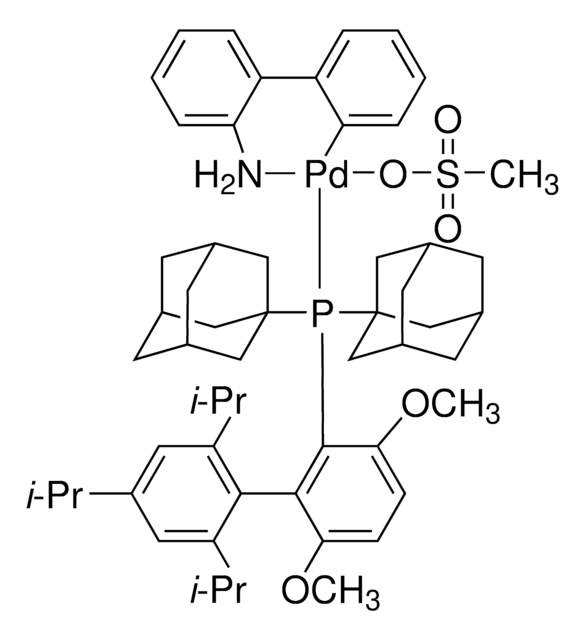
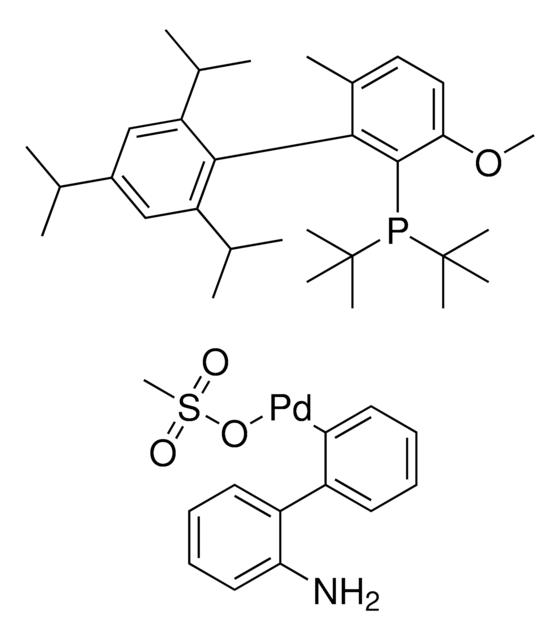
761435
cataCXium® A Pd G3
95%
Sinónimos:
Mesylate[(di(1-adamantyl)-n-butylphosphine)-2-(2′-amino-1,1′-biphenyl)]palladium(II), [(Di(1-adamantyl)-butylphosphine)-2-(2′-amino-1,1′-biphenyl)]palladium(II) methanesulfonate, cataCXium-A-Pd-G3
About This Item
Productos recomendados
Nivel de calidad
Ensayo
95%
Formulario
solid
Características
generation 3
idoneidad de la reacción
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
reaction type: Cross Couplings
puntuación de productos alternativos más sostenibles
old score: 16
new score: 2
Find out more about DOZN™ Scoring
características de los productos alternativos más sostenibles
Waste Prevention
Atom Economy
Safer Solvents and Auxiliaries
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
impurezas
≤3% acetone
mp
196-241 °C (decomposition)
grupo funcional
phosphine
categoría alternativa más sostenible
cadena SMILES
CS(=O)(=O)O[Pd]c1ccccc1-c2ccccc2N.CCCCP([C@@]34C[C@@H]5C[C@@H](C[C@@H](C5)C3)C4)[C@@]67C[C@@H]8C[C@@H](C[C@@H](C8)C6)C7
InChI
1S/C24H39P.C12H10N.CH4O3S.Pd/c1-2-3-4-25(23-11-17-5-18(12-23)7-19(6-17)13-23)24-14-20-8-21(15-24)10-22(9-20)16-24;13-12-9-5-4-8-11(12)10-6-2-1-3-7-10;1-5(2,3)4;/h17-22H,2-16H2,1H3;1-6,8-9H,13H2;1H3,(H,2,3,4);/q;;;+1/p-1/t17-,18+,19-,20-,21+,22-,23-,24-;;;
Clave InChI
REYVZCOGMIXVNX-DVBMAMJVSA-M
Descripción general
Aplicación
- Direct ortho-arylation of pyridinecarboxylic acids.
- Catalyzing Suzuki–Miyaura cross-coupling in the synthesis of 1-heteroaryl-3-azabicyclo[3.1.0]hexanes.
- Palladium-catalyzed carbonylative carboperfluoroalkylation of alkynes.
- Suzuki–Miyaura coupling reaction of geminal bis(boryl)cyclopropanes in the synthesis of various gem-disubstituted cyclopropanes.
- Boroperfluoroalkylation of terminal alkynes.
- Copper-free Sonogashira coupling reaction of aromatic halides with alkynes to form C-C bond.
- Suzuki cross-coupling between organotrifluoroborate and aryl halides.
Información legal
Código de clase de almacenamiento
13 - Non Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
All of the preformed catalysts used in the kit are air and moisture stable complexes in their commercially available form.
Tools aid in kit setup for organic chemistry techniques, ensuring ease and success.
Tools aid in kit setup for organic chemistry techniques, ensuring ease and success.
Tools aid in kit setup for organic chemistry techniques, ensuring ease and success.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico




![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)

![[(1,3,5,7-Tetramethyl-6-phenyl-2,4,6-trioxa-6-phosphaadamantane)-2-(2′-amino-1,1′-biphenyl)]palladium(II) methanesulfonate](/deepweb/assets/sigmaaldrich/product/structures/324/001/3ffb4bd2-9c6b-451c-80ee-a217f03ca932/640/3ffb4bd2-9c6b-451c-80ee-a217f03ca932.png)