392189
1-Pyreneacetic acid
97%
Sinónimos:
(1-Pyrenyl)acetic acid
About This Item
Productos recomendados
assay
97%
form
solid
mp
210-212 °C (dec.) (lit.)
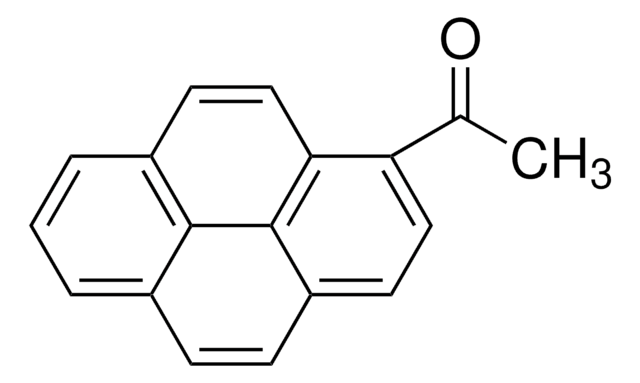
SMILES string
OC(=O)Cc1ccc2ccc3cccc4ccc1c2c34
InChI
1S/C18H12O2/c19-16(20)10-14-7-6-13-5-4-11-2-1-3-12-8-9-15(14)18(13)17(11)12/h1-9H,10H2,(H,19,20)
InChI key
SDJCLYBBPUHKCD-UHFFFAOYSA-N
General description
Application
- Synthesis of N-(2-(methylthio)ethyl)-2-(pyren-1-yl)acetamide, a pyrene amide based Pd2+ probe.
- Synthesis of pyrene-modified β-cyclodextrin.
- To functionalize single walled carbon nanotube field effect transistors (CNT FETs).
- As an agent for characterizing grafting degrees and reactivity of the ester functionalized polypropylenes.
- Synthesis sawhorse-type diruthenium tetracarbonyl complexes.
- Synthesis of (±)-2-(1-pyrenyl)propionic acid, a chiral carboxylic acid.
- Reversible noncovalent functionalization of single walled carbon nanotubes (SWNTs).
- Preparation of peptide nucleic acid (PNA) probes.
- As an internal reference compound in the assessment of solid phase reaction by HPLC-UV.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico