A7902
2-Amino-2-norbornanecarboxylic acid
suitable for ligand binding assays
Synonyme(s) :
BCH, 2-Aminobicyclo[2.2.1]heptane-2-carboxylic acid
About This Item
Produits recommandés
product name
2-Amino-2-norbornanecarboxylic acid, amino acid transport inhibitor
Niveau de qualité
Forme
powder
Technique(s)
ligand binding assay: suitable
Couleur
white to off-white
Pf
>300 °C (lit.)
Chaîne SMILES
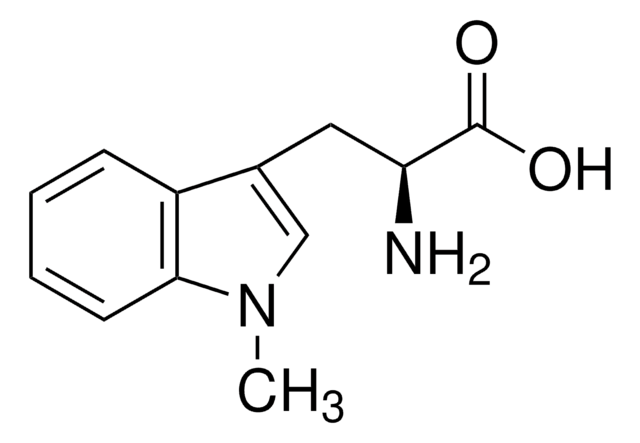
NC1(C[C@@H]2CC[C@H]1C2)C(O)=O
InChI
1S/C8H13NO2/c9-8(7(10)11)4-5-1-2-6(8)3-5/h5-6H,1-4,9H2,(H,10,11)/t5-,6+,8?/m1/s1
Clé InChI
MPUVBVXDFRDIPT-RSHNMJPRSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- Thyroid Hormone Transporters in a Human Placental Cell Model.: This study investigates the role of 2-Amino-2-norbornanecarboxylic acid in the transport of thyroid hormones in placental cells, offering insights that could improve understanding of fetal development and maternal health (Chen et al., 2022).
- Metabolic adaptations to hypoxia in the neonatal mouse forebrain can occur independently of the transporters SLC7A5 and SLC3A2.: Research explores how 2-Amino-2-norbornanecarboxylic acid affects metabolic responses to hypoxia in neonatal brain development, providing a foundation for future studies on brain health and neurodevelopment (Fitzgerald et al., 2021).
- Hemocompatible LAT1-inhibitor can induce apoptosis in cancer cells without affecting brain amino acid homeostasis.: This article assesses the potential of 2-Amino-2-norbornanecarboxylic acid as a selective inhibitor that could lead to new treatments for cancer while preserving critical brain functions (Markowicz-Piasecka et al., 2020).
- Regulation of Melanogenesis by the Amino Acid Transporter SLC7A5.: Research demonstrates the utility of 2-Amino-2-norbornanecarboxylic acid in regulating skin pigmentation processes, which could have implications for disorders related to pigmentation (Gaudel et al., 2020).
- Structure of the human LAT1-4F2hc heteromeric amino acid transporter complex.: This study utilizes 2-Amino-2-norbornanecarboxylic acid to elucidate the structure of a key amino acid transporter, which is crucial for understanding nutrient uptake and its implications in various diseases (Yan et al., 2019).
Actions biochimiques/physiologiques
Qualité
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique