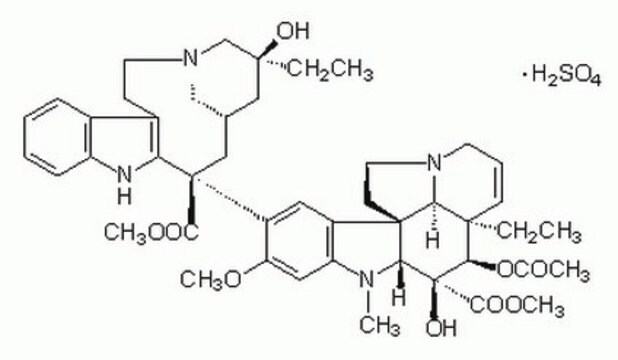
The minimum purity of this Vinblastine Sulfate product is 96%. The exact purity is lot specific and reported on the product Certificate of Analysis. Maximum limits allowable are also set for several residual solvents and related substances. Total impurities may not exceed 3%. The H2SO4 makes up 10.7% of the purified compound based on the molecular formula: C46H58N4O9 · H2SO4. Please see the link below to access a sample or lot specific Certificate:
https://www.sigmaaldrich.com/product/sigma/v1377#product-documentation
V1377
Vinblastine sulfate salt
≥97% (HPLC), powder, plant alkaloid
Sinónimos:
VLB, Vincaleukoblastine sulfate salt
Seleccione un Tamaño
Seleccione un Tamaño
About This Item
Productos recomendados
Nombre del producto
Vinblastine sulfate salt, ≥97% (HPLC)
Nivel de calidad
Ensayo
≥97% (HPLC)
Formulario
(powder or amorphous or crystalline powder)
color
white to light yellow
mp
267 °C (dec.) (lit.)
Absorción
14 at 270 nm in 0.1 M phosphate buffer at 1 mM
16.2 at 259 nm in ethanol at 1 mM
53.7 at 214 nm in ethanol at 1 mM
espectro de actividad antibiótica
neoplastics
Modo de acción
DNA synthesis | interferes
emisor
Eli Lilly
temp. de almacenamiento
2-8°C
cadena SMILES
OS(O)(=O)=O.[H][C@@]12CN(CCc3c([nH]c4ccccc34)[C@@](C1)(C(=O)OC)c5cc6c(cc5OC)N(C)[C@@]7([H])[C@](O)([C@H](OC(C)=O)[C@]8(CC)C=CCN9CC[C@]67[C@]89[H])C(=O)OC)C[C@](O)(CC)C2
InChI
1S/C46H58N4O9.H2O4S/c1-8-42(54)23-28-24-45(40(52)57-6,36-30(15-19-49(25-28)26-42)29-13-10-11-14-33(29)47-36)32-21-31-34(22-35(32)56-5)48(4)38-44(31)17-20-50-18-12-16-43(9-2,37(44)50)39(59-27(3)51)46(38,55)41(53)58-7;1-5(2,3)4/h10-14,16,21-22,28,37-39,47,54-55H,8-9,15,17-20,23-26H2,1-7H3;(H2,1,2,3,4)/t28-,37-,38+,39+,42-,43+,44+,45-,46-;/m0./s1
Clave InChI
KDQAABAKXDWYSZ-PNYVAJAMSA-N
Información sobre el gen
human ... TBCC(6903) , TUBA1A(7846) , TUBA1B(10376) , TUBA1C(84790) , TUBA3C(7278) , TUBA3E(112714) , TUBA4A(7277) , TUBB(203068) , TUBB1(81027) , TUBB2A(7280) , TUBB2B(347733) , TUBB3(10381) , TUBB4A(10382) , TUBB4B(10383) , TUBB6(84617) , TUBB8(347688)
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Aplicación
- as a microtubule depolymerizing drug for the synchronization of human cell lines in G2/M phase[1]
- as a multidrug resistance screening substrate in human colon cancer cell line (HCT116) cell line[2]
- as an antimicrotubule agent in sub perineural glia of Drosophila brain[3]
Acciones bioquímicas o fisiológicas
Características y beneficios
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 4 Oral - Muta. 2 - Repr. 2
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Discover Bioactive Small Molecules for ADME/Tox
-
What is the composition of vinblastine sulfate salt. I mean what is the ratio of vinblastine: salt in this powder
1 answer-
Helpful?
-
Active Filters
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico