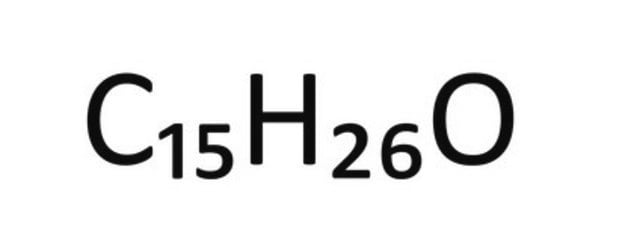W277207
Nerolidol
mixture of cis and trans, ≥97%, stabilized, FG
Sinónimos:
3,7,11-Trimethyl-1,6,10-dodecatrien-3-ol, 3-Hydroxy-3,7,11-trimethyl-1,6,10-dodecatriene
About This Item
Productos recomendados
biological source
synthetic
Quality Level
grade
FG
Kosher
agency
meets purity specifications of JECFA
reg. compliance
EU Regulation 1334/2008 & 872/2012
FCC
FDA 21 CFR 172.515
assay
≥97%
contains
synthetic α-tocopherol as stabilizer
refractive index
n20/D 1.479 (lit.)
bp
114 °C/1 mmHg (lit.)
density
0.875 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
documentation
see Safety & Documentation for available documents
food allergen
no known allergens
organoleptic
green; floral; woody
SMILES string
C\C(C)=C/CC\C(C)=C\CCC(C)(O)C=C
InChI
1S/C15H26O/c1-6-15(5,16)12-8-11-14(4)10-7-9-13(2)3/h6,9,11,16H,1,7-8,10,12H2,2-5H3/b14-11+
InChI key
FQTLCLSUCSAZDY-SDNWHVSQSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
- Effects of Six Natural Compounds and Their Derivatives on the Control of Coccidiosis in Chickens.: This article discusses the use of natural compounds including nerolidol for controlling coccidiosis in poultry, indicating its applications in veterinary biochemistry and animal health (Hou et al., 2024).
signalword
Warning
hcodes
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Skin Sens. 1
Storage Class
10 - Combustible liquids
wgk_germany
WGK 2
flash_point_f
262.4 °F - closed cup
flash_point_c
128 °C - closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico