449504
Bis(trifluorometano)sulfonimida lithium salt
Sinónimos:
Bis(trifluorometilsulfonil)amina lithium salt, Bistrifluorometanosulfonimidato de litio
About This Item
Productos recomendados
mp
234-238 °C (lit.)
grupo funcional
fluoro
cadena SMILES
[Li]N(S(=O)(=O)C(F)(F)F)S(=O)(=O)C(F)(F)F
InChI
1S/C2F6NO4S2.Li/c3-1(4,5)14(10,11)9-15(12,13)2(6,7)8;/q-1;+1
Clave InChI
QSZMZKBZAYQGRS-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Aplicación
- As a chemical additive for improving the power conversion efficiencies in porphyrin-based organic solar cells.
- As a reagent in the preparation of imidazolium core bearing monomer ionic liquids to develop polymerized ionic liquids.
- For the preparation of a chiral imidazolium salt via anion metathesis of the corresponding triflate.
- In the synthesis of solid polymer electrolytes for lithium-ion batteries.
- In the synthesis of polyelectrolyte reusable homogenous catalysts, which are used in the Diels–Alder reactions between isoprene and a variety of dienophiles.
- Used in the preparation of electrolytes for lithium batteries and novel rare-earth Lewis acid catalysts.
Otras notas
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 3 Dermal - Acute Tox. 3 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1B - STOT RE 2 Oral
Órganos de actuación
Nervous system
Código de clase de almacenamiento
6.1B - Non-combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Experts discuss challenges and production processes of nickel-rich layered oxide cathode materials in energy storage systems.
Lithium-ion batteries offer high energy density and cyclic performance for portable electronic devices.
The critical technical challenges associated with the commercialization of electric vehicle batteries include cost, performance, abuse tolerance, and lifespan.
Due to the adverse impact of the continued use of fossil fuels on the earth’s environment and climate, researchers have been asked to develop new approaches for producing power using renewable sources like wind and solar energy
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico





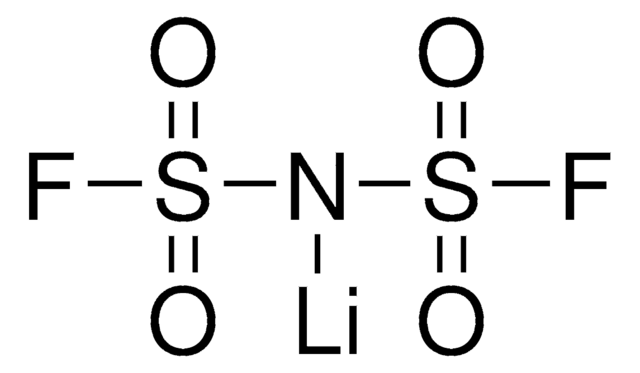




![Zinc di[bis(trifluoromethylsulfonyl)imide] 95%](/deepweb/assets/sigmaaldrich/product/structures/336/073/952daadd-0a7c-4bec-bbaf-442a24c62161/640/952daadd-0a7c-4bec-bbaf-442a24c62161.png)