161179
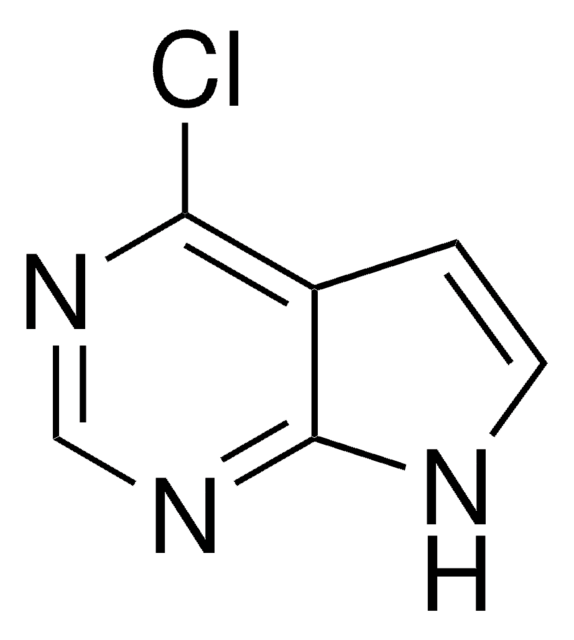
6-Chloropurine
≥99%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C5H3ClN4
Número de CAS:
Peso molecular:
154.56
Beilstein/REAXYS Number:
5774
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
≥99%
form
powder
mp
>300 °C (dec.) (lit.)
solubility
DMF: soluble 5%, clear, colorless to yellow
SMILES string
Clc1ncnc2[nH]cnc12
InChI
1S/C5H3ClN4/c6-4-3-5(9-1-7-3)10-2-8-4/h1-2H,(H,7,8,9,10)
InChI key
ZKBQDFAWXLTYKS-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
The acid-catalyzed reaction of 6-chloropurine with 3,4-di-O-acetyl-D-xylal has been investigated.
Application
6-Chloropurine has been used in the preparation of 9-alkylpurines via alkylation with various substituted alkyl halides in DMSO. It was also used in the preparation of 6-succinoaminopurine.
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Synthesis of 6-succinoaminopurine.
C E CARTER
The Journal of biological chemistry, 223(1), 139-146 (1956-11-01)
Synthesis of Potential Anticancer Agents. XXVI. The Alkylation of 6-Chloropurine2.
Montgomery JA and Temple Jr C.
Journal of the American Chemical Society, 83(3), 630-635 (1961)
Prashantha Gunaga et al.
The Journal of organic chemistry, 69(9), 3208-3211 (2004-04-24)
Novel thioiso pyrimidine and purine nucleosides substituted with exocyclic methylene have been synthesized, starting from D-xylose. The glycosyl donor 14 was synthesized from D-xylose, using cyclization of dimesylate 10 with sodium sulfide as a key step. Cyclization proceeded in pure
Michal Sála et al.
Bioorganic & medicinal chemistry letters, 22(5), 1963-1968 (2012-02-09)
We report on the synthesis and the study of the structure-activity relationship of novel 9-norbornyl-6-chloropurine derivatives, which exert selective antiviral activity on the replication of Coxsackievirus B3. In particular, the synthetic approaches towards norbornyl derivatives bearing diverse side chains were
Shi Bai et al.
Magnetic resonance in chemistry : MRC, 48(1), 61-67 (2009-11-26)
The (15)N and (13)C chemical shifts of 6-(fluoro, chloro, bromo, and iodo)purine 2'-deoxynucleoside derivatives in deuterated chloroform were measured. The (15)N chemical shifts were determined by the (1)H-(15)N HMBC method, and complete (15)N chemical-shift assignments were made with the aid
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico