138827
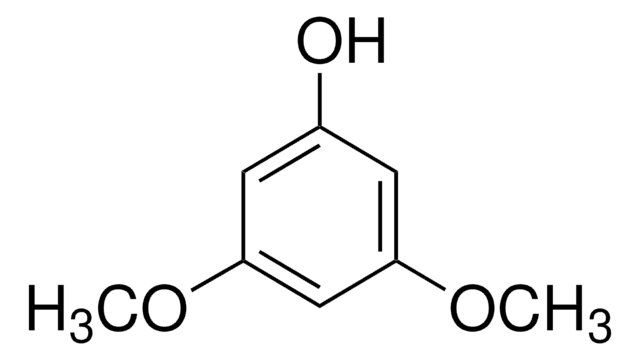
1,3,5-Trimethoxybenzene
ReagentPlus®, ≥99%
Sinónimos:
Phloroglucinol trimethyl ether
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
C6H3(OCH3)3
Número de CAS:
Peso molecular:
168.19
Beilstein:
1307993
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
Nivel de calidad
Línea del producto
ReagentPlus®
Ensayo
≥99%
Formulario
solid
bp
255 °C (lit.)
mp
50-53 °C (lit.)
cadena SMILES
COc1cc(OC)cc(OC)c1
InChI
1S/C9H12O3/c1-10-7-4-8(11-2)6-9(5-7)12-3/h4-6H,1-3H3
Clave InChI
LKUDPHPHKOZXCD-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
1,3,5-Trimethoxybenzene effectively cleaves p-methoxybenzyl protecting group on various alcohols and acids. It is the major scent compound present in Chinese rose species.
Aplicación
1,3,5-Trimethoxybenzene was used to study the photodeoxygenation of 1,2-benzodiphenylene sulfoxide. It was employed as secondary standard in quantitative proton NMR spectroscopy of pharmaceuticals.
Información legal
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 4 Oral
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nicolas Kern et al.
The Journal of organic chemistry, 77(20), 9227-9235 (2012-09-26)
The p-methoxybenzyl protecting group (PMB) on various alcohols and an acid was efficiently and selectively cleaved by the action of a catalytic amount of silver(I) hexafluoroantimonate combined with 0.5 equiv of 1,3,5-trimethoxybenzene in dichloromethane at 40 °C.
Xichen Cai et al.
The journal of physical chemistry. A, 111(22), 4743-4747 (2007-05-08)
Bimolecular hole transfer quenching of the 1,3,5-trimethoxybenzene radical cation (TMB*+) in the excited state (TMB*+*) by hole quenchers (Q) such as biphenyl (Bp), naphthalene (Np), anisole (An), and benzene (Bz) with higher oxidation potentials than that of TMB was directly
O Chassany et al.
Alimentary pharmacology & therapeutics, 25(9), 1115-1123 (2007-04-19)
Abdominal pain is the predominant symptom in irritable bowel syndrome patients. Phloroglucinol and its methylated derivative are antispasmodic agents acting on smooth muscle. To evaluate the efficacy of phloroglucinol/trimethylphloroglucinol on pain intensity during an acute exacerbation of pain of irritable
Tao Fang et al.
Journal of the American Chemical Society, 134(17), 7545-7552 (2012-04-06)
The development of selectively protected monosaccharide building blocks that can reliably be glycosylated with a wide variety of acceptors is expected to make oligosaccharide synthesis a more routine operation. In particular, there is an urgent need for the development of
Xichen Cai et al.
The journal of physical chemistry. A, 111(10), 1788-1791 (2007-02-14)
One-electron oxidation of alcohols such as methanol, ethanol, and 2-propanol by 1,3,5-trimethoxybenzene radical cation (TMB*+) in the excited state (TMB*+*) was observed during the two-color two-laser flash photolysis. TMB*+ was formed by the photoinduced bimolecular electron-transfer reaction from TMB to
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico