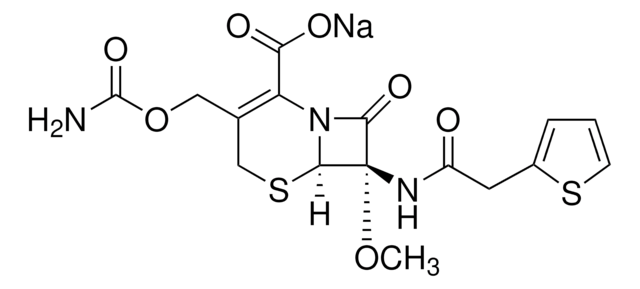
C4786
Cefoxitin sodium salt
About This Item
Prodotti consigliati
Forma fisica
powder or granules
Livello qualitativo
tecniche
HPLC: suitable
gas chromatography (GC): suitable
applicazioni
forensics and toxicology
pharmaceutical (small molecule)
veterinary
Temperatura di conservazione
2-8°C
Stringa SMILE
[Na+].CO[C@]2(NC(=O)Cc1cccs1)C3SCC(COC(N)=O)=C(N3C2=O)C([O-])=O
InChI
1S/C16H17N3O7S2.Na/c1-25-16(18-10(20)5-9-3-2-4-27-9)13(23)19-11(12(21)22)8(6-26-15(17)24)7-28-14(16)19;/h2-4,14H,5-7H2,1H3,(H2,17,24)(H,18,20)(H,21,22);/q;+1/p-1/t14-,16+;/m1./s1
GNWUOVJNSFPWDD-XMZRARIVSA-M
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Applicazioni
- Determine cefoxitin sodium using four stability-indicating spectrophotometric methods in the presence of its alkaline degradation products in its pure form and pharmaceutical dosage forms
- Separate and measure cefoxitin sodium in commercial drug products by high-performance liquid chromatography (HPLC)
Altre note
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Skin Sens. 1B
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Choose from one of the most recent versions:
Certificati d'analisi (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.