910449
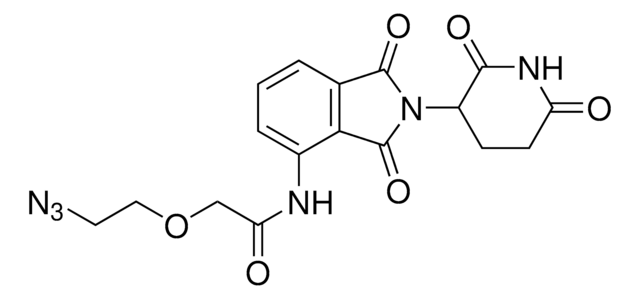
Pomalidomide-PEG2-butyl CO2H
≥95%
Sinonimo/i:
7-(2-(2-((2-(2,6-Dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)amino)-2-oxoethoxy)ethoxy)heptanoic acid, Crosslinker−E3 ligase ligand conjugate, Pomalidomide conjugate, Pomalidomide-2-2-6-acid, Protein degrader building block for PROTAC® research, Template for synthesis of targeted protein degrader
About This Item
Prodotti consigliati
ligand
pomalidomide
Saggio
≥95%
Forma fisica
powder
Impiego in reazioni chimiche
reactivity: amine reactive
reagent type: ligand-linker conjugate
Gruppo funzionale
carboxylic acid
Temperatura di conservazione
2-8°C
Stringa SMILE
O=C(C(CC1)N(C2=O)C(C3=C2C=CC=C3NC(COCCOCCCCCCC(O)=O)=O)=O)NC1=O
Applicazioni
Altre note
Portal: Building PROTAC® Degraders for Targeted Protein Degradation
Targeted Protein Degradation by Small Molecules
Small-Molecule PROTACS: New Approaches to Protein Degradation
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
Impact of linker length on the activity of PROTACs
Note legali
Prodotti correlati
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Repr. 1B
Codice della classe di stoccaggio
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Articoli
Protein Degrader Building Blocks are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.