901116
trans-Bis(dicyclohexylphenylphosphine)(2-methylphenyl)nickel(II) chloride
Sinonimo/i:
trans-(PCy2Ph)2Ni(o-tolyl)Cl
About This Item
Prodotti consigliati
Forma fisica
powder or solid
Impiego in reazioni chimiche
core: nickel
reaction type: Cross Couplings
reagent type: catalyst
Punto di fusione
174-179 °C
Stringa SMILE
Cl[Ni](P(C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C1([H])[H])(C2=C([H])C([H])=C([H])C([H])=C2[H])C3([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C3([H])[H])(P(C4([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C4([H])[H])(C5=C([H])C([H])=C([H
Altre note
Note legali
Prodotti correlati
Codice della classe di stoccaggio
13 - Non Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Articoli
The Jamison group has developed a library of bench-stable phosphine-containing nickel(II) precatalysts that are converted into active catalysts in situ.
Nickel complexes catalyze various synthetic reactions like oxidative addition, C-H activation, and cross-coupling.
Contenuto correlato
Research in the Jamison group is centered on the development of new reactions and technologies for organic synthesis. Towards these themes, the group has pioneered a number of air-stable nickel precatalysts supported by phosphines and N-heterocyclic carbenes that are readily converted to the active catalyst in situ.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.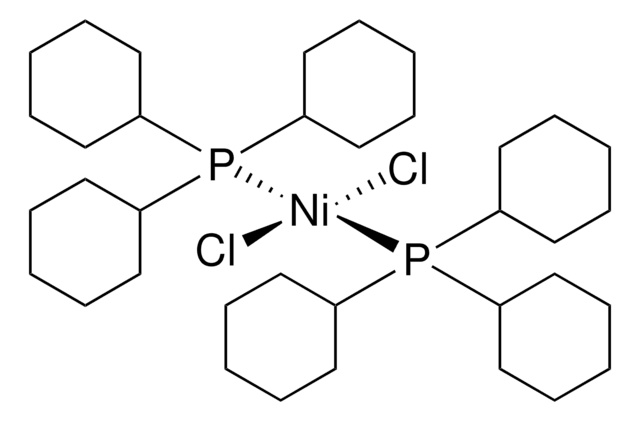

nickel(II) chloride](/deepweb/assets/sigmaaldrich/product/structures/252/197/3c560697-beb3-4c52-85d6-ebc3af13db69/640/3c560697-beb3-4c52-85d6-ebc3af13db69.png)
![[(TMEDA)Ni(o-tolyl)Cl] 95%](/deepweb/assets/sigmaaldrich/product/structures/236/439/768c916e-994f-47e3-a980-3ca0471317d7/640/768c916e-994f-47e3-a980-3ca0471317d7.png)
![[1,2-Bis(diphenylphosphino)ethane]dichloronickel(II)](/deepweb/assets/sigmaaldrich/product/structures/707/956/483e7a6e-5fb5-4e39-abd1-ecf33ccab3cf/640/483e7a6e-5fb5-4e39-abd1-ecf33ccab3cf.png)
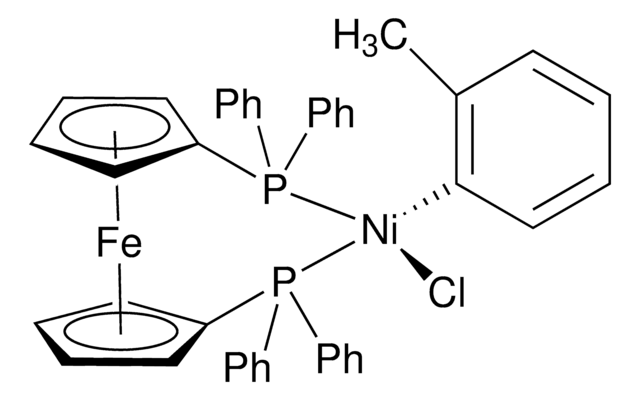
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloronickel(II) 97%](/deepweb/assets/sigmaaldrich/product/structures/274/566/a60d6584-163a-4c41-a738-60f8e4d524fa/640/a60d6584-163a-4c41-a738-60f8e4d524fa.png)