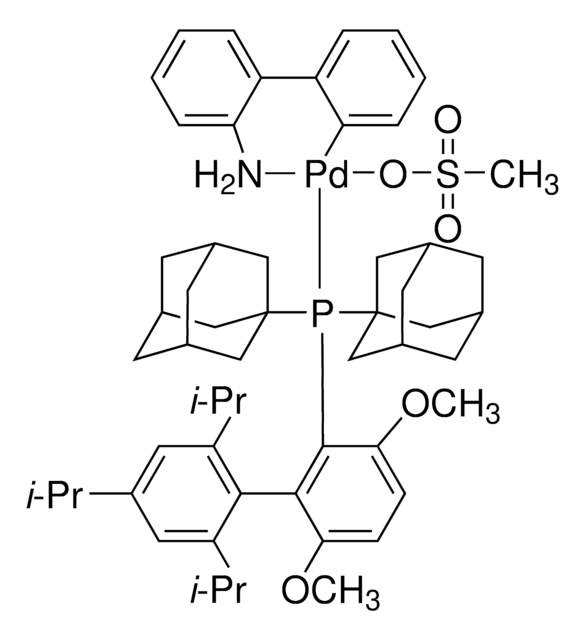
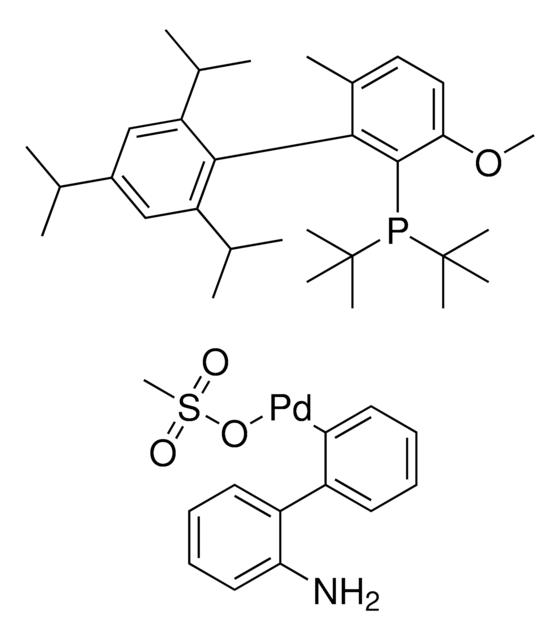
761435
cataCXium® A Pd G3
95%
Sinonimo/i:
Mesylate[(di(1-adamantyl)-n-butylphosphine)-2-(2′-amino-1,1′-biphenyl)]palladium(II), [(Di(1-adamantyl)-butylphosphine)-2-(2′-amino-1,1′-biphenyl)]palladium(II) methanesulfonate, cataCXium-A-Pd-G3
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
95%
Forma fisica
solid
Caratteristiche
generation 3
Impiego in reazioni chimiche
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
reaction type: Cross Couplings
Punteggio alternativa più verde
old score: 16
new score: 2
Find out more about DOZN™ Scoring
Caratteristiche più verdi
Waste Prevention
Atom Economy
Safer Solvents and Auxiliaries
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Impurezze
≤3% acetone
Punto di fusione
196-241 °C (decomposition)
Gruppo funzionale
phosphine
Categoria alternativa più verde
Stringa SMILE
CS(=O)(=O)O[Pd]c1ccccc1-c2ccccc2N.CCCCP([C@@]34C[C@@H]5C[C@@H](C[C@@H](C5)C3)C4)[C@@]67C[C@@H]8C[C@@H](C[C@@H](C8)C6)C7
InChI
1S/C24H39P.C12H10N.CH4O3S.Pd/c1-2-3-4-25(23-11-17-5-18(12-23)7-19(6-17)13-23)24-14-20-8-21(15-24)10-22(9-20)16-24;13-12-9-5-4-8-11(12)10-6-2-1-3-7-10;1-5(2,3)4;/h17-22H,2-16H2,1H3;1-6,8-9H,13H2;1H3,(H,2,3,4);/q;;;+1/p-1/t17-,18+,19-,20-,21+,22-,23-,24-;;;
REYVZCOGMIXVNX-DVBMAMJVSA-M
Descrizione generale
Applicazioni
- Direct ortho-arylation of pyridinecarboxylic acids.
- Catalyzing Suzuki–Miyaura cross-coupling in the synthesis of 1-heteroaryl-3-azabicyclo[3.1.0]hexanes.
- Palladium-catalyzed carbonylative carboperfluoroalkylation of alkynes.
- Suzuki–Miyaura coupling reaction of geminal bis(boryl)cyclopropanes in the synthesis of various gem-disubstituted cyclopropanes.
- Boroperfluoroalkylation of terminal alkynes.
- Copper-free Sonogashira coupling reaction of aromatic halides with alkynes to form C-C bond.
- Suzuki cross-coupling between organotrifluoroborate and aryl halides.
Note legali
Codice della classe di stoccaggio
13 - Non Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
All of the preformed catalysts used in the kit are air and moisture stable complexes in their commercially available form.
Tools aid in kit setup for organic chemistry techniques, ensuring ease and success.
Materials Included in your KITALYSIS-24PD-2PK High-Throughput Screening Kit
G3 and G4 Buchwald palladium precatalysts are the newest air, moisture, and thermally stable crossing-coupling complexes used in bond formation for their versatility and high reactivity.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.




![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)

![[(1,3,5,7-Tetramethyl-6-phenyl-2,4,6-trioxa-6-phosphaadamantane)-2-(2′-amino-1,1′-biphenyl)]palladium(II) methanesulfonate](/deepweb/assets/sigmaaldrich/product/structures/324/001/3ffb4bd2-9c6b-451c-80ee-a217f03ca932/640/3ffb4bd2-9c6b-451c-80ee-a217f03ca932.png)