372404
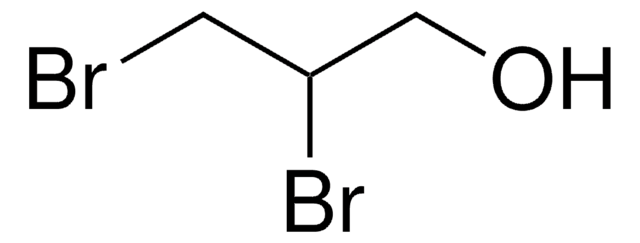
1,3-Dibromo-2-propanol
technical grade, 95%
Sinonimo/i:
α,γ-Dibromohydrin, α-Dibromohydrin, 1,3-Dibromo-2-hydroxypropane, 1,3-Dibromohydrin, 1,3-Dibromopropanol, 2-Hydroxy-1,3-dibromopropane, Glycerol α,γ-dibromohydrin, Glycerol 1,3-dibromohydrin
Scegli un formato
Scegli un formato
About This Item
Prodotti consigliati
Grado
technical grade
Livello qualitativo
Saggio
95%
Stato
liquid
Indice di rifrazione
n20/D 1.552 (lit.)
P. ebollizione
82-83 °C/7 mmHg (lit.)
Densità
2.136 g/mL at 25 °C (lit.)
Gruppo funzionale
bromo
hydroxyl
Stringa SMILE
OC(CBr)CBr
InChI
1S/C3H6Br2O/c4-1-3(6)2-5/h3,6H,1-2H2
KIHQZLPHVZKELA-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Applicazioni
- To prepare the thioether ligand, used in the synthesis of palladium-phosphorus/sulfur nanoparticles.[2]
- Preparation of 2-(alkoxy)propenyl bromide.[3]
- Synthesis of 1,3-propanediamine derivatives connected to carbohydrates (sugar-pendant diamines).[4]
- Chemical crosslinking of nine single substitution cysteine mutants of staphylococcal nuclease.[1]
- Preparation of 1,3-dinitrooxy-2-propanol, via reaction with AgNO3 in MeCN at 80°C.[5]
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 3 Oral - Carc. 2 - Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
3 - Flammable liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
116.6 °F - closed cup
Punto d’infiammabilità (°C)
47 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Active Filters
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.