139858
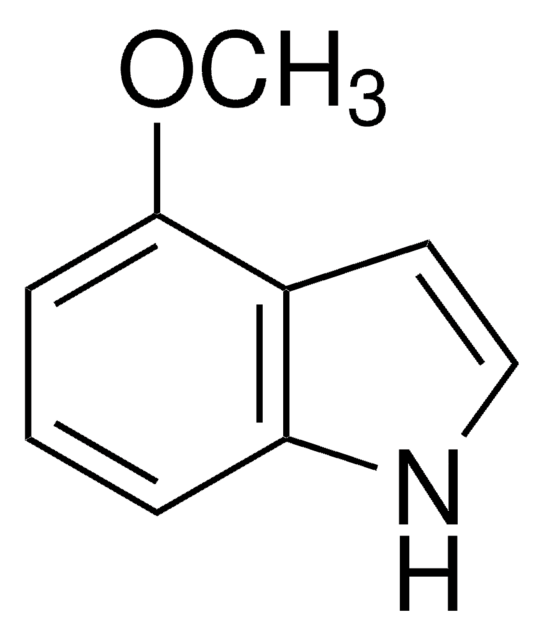
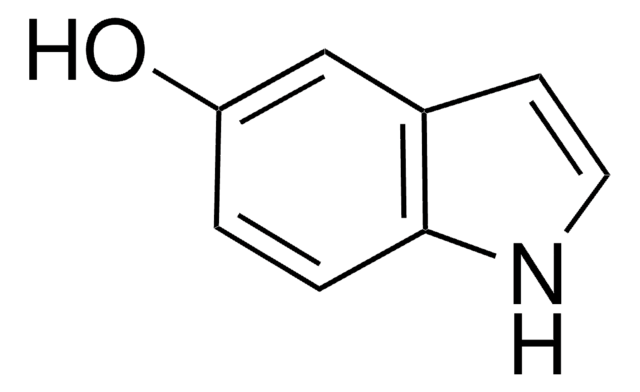
6-Methoxyindole
98%
Sinonimo/i:
NSC 92517
About This Item
Prodotti consigliati
Saggio
98%
Punto di fusione
90-92 °C (lit.)
Stringa SMILE
COc1ccc2cc[nH]c2c1
InChI
1S/C9H9NO/c1-11-8-3-2-7-4-5-10-9(7)6-8/h2-6,10H,1H3
QJRWYBIKLXNYLF-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
- tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles, potential anticancer immunomodulator
- indolylindazoles and indolylpyrazolopyridines, interleukin-2 inducible T cell kinase inhibitors
- diindolyloxyindoles, anticancer agents
- benzoylpiperazinyl-indolyl ethane dione derivatives, HIV-1 inhibitors
- 3-aroylindoles as anticancer agents
- indolyl and isoquinolinyl anthranilates, PPARδ partial agonists
- heteroaryl ketones, VEGFR-2 inhibitors
- Reactant for preparation of tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles as potential anticancer immunomodulators
- Reactant for synthesis of indolylindazoles and indolylpyrazolopyridines as interleukin-2 inducible T cell kinase inhibitors
- Reactant for preparation of diindolyloxyindoles as anticancer agents
- Reactant for preparation of benzoylpiperazinyl-indolyl ethane dione derivatives as HIV-1 inhibitors
- Reactant for preparation of 3-aroylindoles as anticancer agents
- Reactant for preparation of indolyl and isoquinolinyl anthranilates as PPARδ partial agonists
- Reactant for preparation of heteroaryl ketones as VEGFR-2 inhibitors
Azioni biochim/fisiol
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.