137561
3-Methyl-1-pentyn-3-ol
98%
Sinonimo/i:
Ethyl ethynyl methyl carbinol, Meparfynol
Scegli un formato
About This Item
Prodotti consigliati
Densità del vapore
3 (vs air)
Livello qualitativo
Tensione di vapore
6.5 mmHg ( 20 °C)
Saggio
98%
Stato
liquid
Indice di rifrazione
n20/D 1.431 (lit.)
P. ebollizione
121-122 °C (lit.)
Solubilità
Cellosolve: miscible
Stoddard solvent: miscible
acetone: miscible
benzene: miscible
carbon tetrachloride: miscible
cyclohexanone: miscible
diethyl ether: soluble
diethylene glycol: miscible
ethanolamine: miscible
ethyl acetate: miscible
kerosene: miscible
mineral spirits: miscible
neatsfoot oil: miscible
petroleum ether: miscible
soybean oil: miscible
Densità
0.866 g/mL at 25 °C (lit.)
Gruppo funzionale
hydroxyl
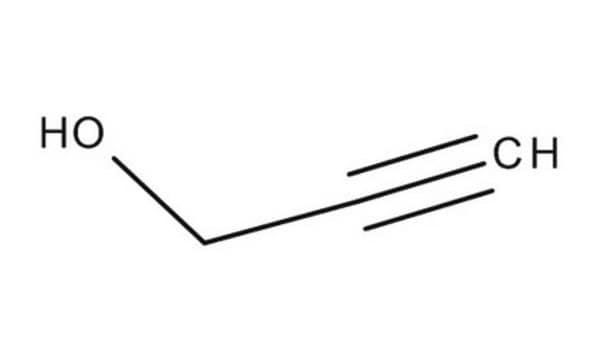
Stringa SMILE
CCC(C)(O)C#C
InChI
1S/C6H10O/c1-4-6(3,7)5-2/h1,7H,5H2,2-3H3
QXLPXWSKPNOQLE-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Applicazioni
- A reactant to synthesize α-methylene cyclic carbonates by reacting with carbon dioxide.[2]
- A reactant in the synthesis of 2,6,9-trisubstituted purine based CDK inhibitors.[3]
- An initiator in the synthesis of polylactide bearing terminal propargyl group via ring-opening polymerization of L-lactide.[1]
Azioni biochim/fisiol
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Flam. Liq. 3
Codice della classe di stoccaggio
3 - Flammable liquids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
82.4 °F - closed cup
Punto d’infiammabilità (°C)
28 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Active Filters
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.