930636
VH 032 amide-alkyl C5-acid
≥95%
Synonym(e):
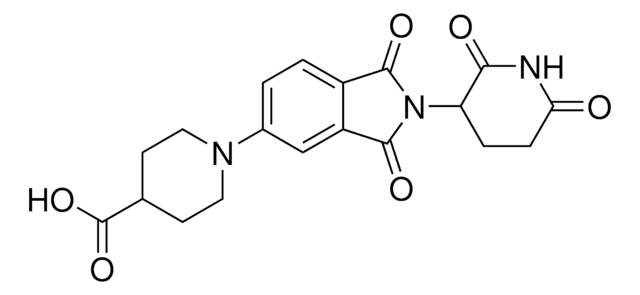
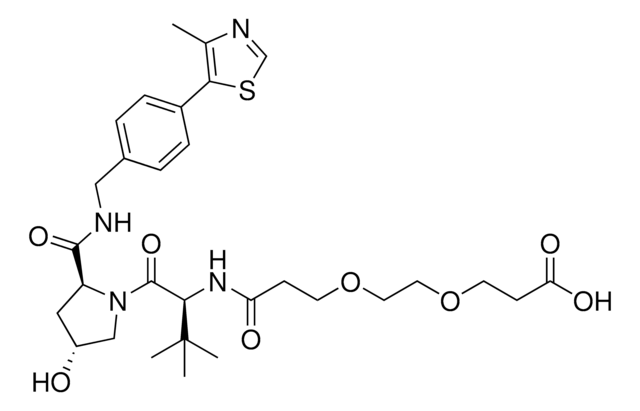
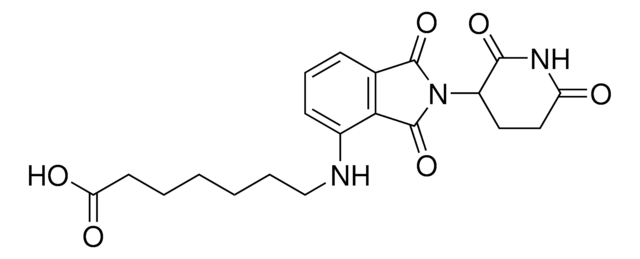
(S,R,S)-AHPC-CO-C5-acid, (S,R,S)-AHPC-amido-C5-acid, 6-{[(2S)-1-[(2S,4R)-4-hydroxy-2-({[4-(4-methyl-1,3-thiazol-5-yl)phenyl]methyl}carbamoyl)pyrrolidin-1-yl]-3,3-dimethyl-1-oxobutan-2-yl]carbamoyl}hexanoic acid, L-Prolinamide, N-(6-carboxy-1-oxohexyl)-3-methyl-L-valyl-4-hydroxy-N-[[4-(4-methyl-5-thiazolyl)phenyl],methyl]-, (4R)-
About This Item
Empfohlene Produkte
ligand
VH032
Qualitätsniveau
Assay
≥95%
Form
powder
Funktionelle Gruppe
carboxylic acid
Lagertemp.
2-8°C
SMILES String
C([C@@H](NC(CCCCCC(O)=O)=O)C(C)(C)C)(=O)N1[C@H](C(NCC2=CC=C(C=C2)C3=C(C)N=CS3)=O)C[C@@H](O)C1
Anwendung
Technology Spotlight: Degrader Building Blocks for Targeted Protein Degradation
Protein Degrader Building Blocks
Sonstige Hinweise
Destruction of DNA-Binding Proteins by Programmable Oligonucleotide PROTAC (O′PROTAC): Effective Targeting of LEF1 and ERG
Small-Molecule PROTACS: New Approaches to Protein Degradation
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
Impact of linker length on the activity of PROTACs
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.