Wichtige Dokumente
907278
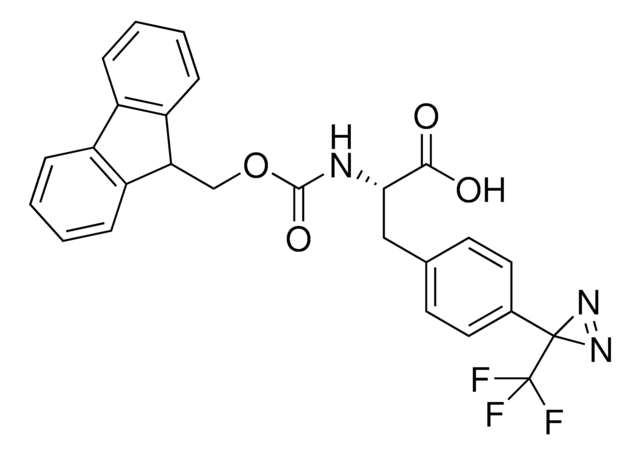
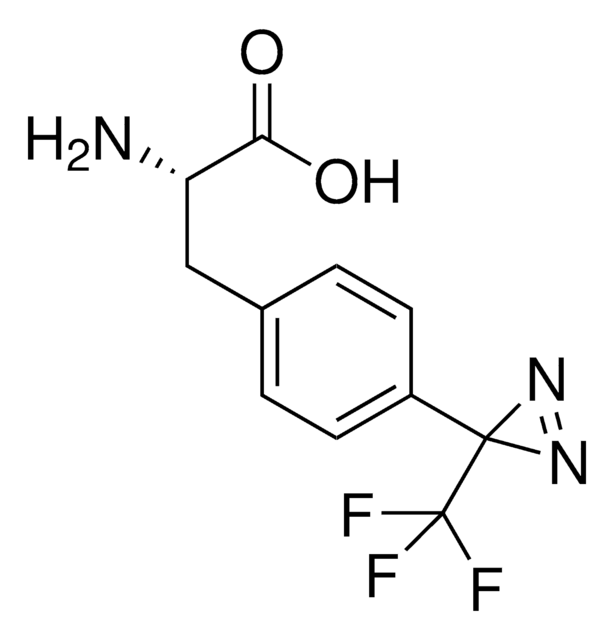
H-L-Photo-leucine HCl
≥98%
Synonym(e):
(S)-2-Amino-3-(3-methyl-3H-diazirin-3-yl)propanoic acid hydrochloride, (S)-2-Amino-3-(3H-diazirin-3-yl)butanoic acid hydrochloride, Diazirine amino acid, Photo-Leu, Photo-crosslinking amino acid, Photoprobe building block
About This Item
Empfohlene Produkte
Assay
≥98%
Form
solid
Eignung der Reaktion
reaction type: solution phase peptide synthesis
Verfügbarkeit
available only in USA
Anwendung(en)
peptide synthesis
Lagertemp.
−20°C
Anwendung
Sonstige Hinweise
for generation of homogeneous conjugates from wild-type antibodies
Mechanistic studies of a small-molecule modulator of SMN2 splicing
Protein-Polymer Conjugation via Ligand Affinity and Photoactivation of Glutathione S-Transferase
Direct Interaction between an Allosteric Agonist Pepducin and the Chemokine Receptor CXCR4
Photo-leucine and photo-methionine allow identification of protein-?protein interactions in living cells
Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis
Photo-affinity labeling (PAL) in chemical proteomics: a handy tool to investigate protein-protein interactions (PPIs)
Ähnliches Produkt
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Self-react. C
Lagerklassenschlüssel
5.2 - Organic peroxides and self-reacting hazardous materials
WGK
WGK 3
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Dokumente section.
Wenn Sie Hilfe benötigen, wenden Sie sich bitte an Kundensupport
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Active Filters
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.