906190
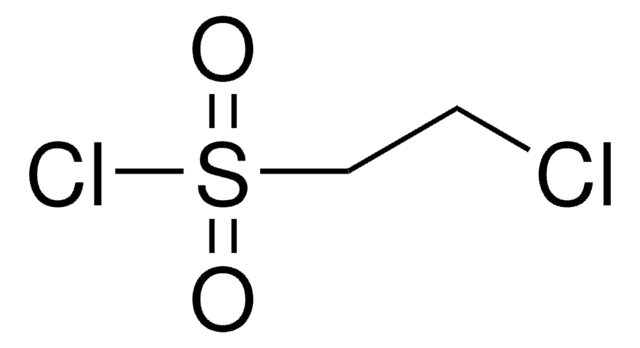
1,2-Dibromoethane-1-sulfonyl fluoride
Synonym(e):
DESF, SuFEx hub, SuFEx-able plugin
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C2H3Br2FO2S
CAS-Nummer:
Molekulargewicht:
269.92
MDL-Nummer:
UNSPSC-Code:
12352101
NACRES:
NA.22
Empfohlene Produkte
Form
liquid
Eignung der Reaktion
reaction type: click chemistry
Verwandte Kategorien
Anwendung
1,2-Dibromoethane-1-sulfonyl fluoride (DESF) is a bench-stable precursor to 1-bromoethene-1-sulfonyl fluoride (BESF), a new and robust connective hub for the Sulfur (VI) fluoride exchange (SuFEx) click reaction. BESF offers similar routes as ethenesulfonyl fluoride (ESF, cat# 746959) but with additional reactivity due to the embedded bromo group.
Treatment of DESF with triethylamine generates BESF in situ, which has been used to synthesize diverse and unprecented sulfonyl fluorides in good-to-excellent yields. As the resulting molecules possess sulfonyl fluoride handles, further SuFEx reactions are also possible. DESF adds another useful tool for conjugation strategies in chemical biology and organic and polymer synthesis.
Automate your fluorination reactions with Synple Automated Synthesis Platform (SYNPLE-SC002)
Treatment of DESF with triethylamine generates BESF in situ, which has been used to synthesize diverse and unprecented sulfonyl fluorides in good-to-excellent yields. As the resulting molecules possess sulfonyl fluoride handles, further SuFEx reactions are also possible. DESF adds another useful tool for conjugation strategies in chemical biology and organic and polymer synthesis.
Automate your fluorination reactions with Synple Automated Synthesis Platform (SYNPLE-SC002)
Ähnliches Produkt
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2
Lagerklassenschlüssel
10 - Combustible liquids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Jing Leng et al.
Chemical communications (Cambridge, England), 54(35), 4477-4480 (2018-04-17)
A new fluorosulfonylation reagent 1-bromoethene-1-sulfonyl fluoride was developed (1-Br-ESF). This unique reagent possesses three addressable handles (vinyl, bromide, and sulfonyl fluoride) and has great potential to function as a tris-electrophile and as a sulfur(vi) fluoride exchange (SuFEx) clickable material to
Christopher J Smedley et al.
Chemical communications (Cambridge, England), 54(47), 6020-6023 (2018-05-26)
We demonstrate 1,2-dibromoethane-1-sulfonyl fluoride (DESF) as a bench-stable and readily accessible precursor to the robust SuFEx connector, 1-bromoethene-1-sulfonyl fluoride (BESF). The in situ generation of BESF from DESF opens up several new reaction profiles, including application in the syntheses of
Joice Thomas et al.
Organic letters, 20(13), 3749-3752 (2018-06-16)
A regioselective metal-free preparation of 4-fluorosulfonyl 1,2,3-triazoles from organic azides and a hitherto underexplored bromovinylsulfonyl fluoride building block is described. This reaction is very general and was extended to the synthesis of various sulfonates, sulfonamides, and sulfonic acid derivatives of
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.