633216
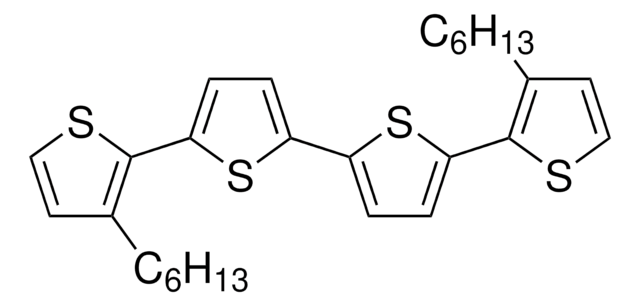
5,5′′′′′-Dihexyl-2,2′:5′,2′′:5′′,2′′′:5′′′,2′′′′:5′′′′,2′′′′′-Sexithiophen
electron donor for OPV devices
Synonym(e):
α,ω-Dihexylsexithiophen, DH-6T
About This Item
Empfohlene Produkte
Form
solid
mp (Schmelzpunkt)
280 °C (dec.) (lit.)
Löslichkeit
chlorobenzene: soluble (soluble)
chloroform: slightly soluble
methylene chloride: slightly soluble
Energie der Orbitale
HOMO 5.2 eV
LUMO 2.9 eV
Leistung von OPV-Bauelementen
ITO/DH6T/PC61BM/Al
ITO/PEDOT:PSS/DH6T/PC61BM/Al
ITO/PEDOT:PSS/DH6T:PC61BM (1:1)/Al
Halbleitereigenschaften
P-type (mobility=0.13 cm2/V·s)
SMILES String
CCCCCCc1ccc(s1)-c2ccc(s2)-c3ccc(s3)-c4ccc(s4)-c5ccc(s5)-c6ccc(CCCCCC)s6
InChI
1S/C36H38S6/c1-3-5-7-9-11-25-13-15-27(37-25)29-17-19-31(39-29)33-21-23-35(41-33)36-24-22-34(42-36)32-20-18-30(40-32)28-16-14-26(38-28)12-10-8-6-4-2/h13-24H,3-12H2,1-2H3
InChIKey
QCMASTUHHXPVGT-UHFFFAOYSA-N
Allgemeine Beschreibung
Anwendung
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Artikel
Review the potential of self-assembled multilayer gate dielectric films fabricated from silane precursors for organic, inorganic, and transparent TFT and for TFT circuitry and OLED displays.
Oligothiophenes are important organic electronic materials which can be produced using synthetic intermediates and Suzuki coupling.
Organic materials in optoelectronic devices like LEDs and solar cells are of significant academic and commercial interest.
Intrinsically stretchable active layers for organic field-effect transistors (OFET) are discussed. Polymer structural modification & post-polymerization modifications are 2 methods to achieve this.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.![[(S)-(−)-1-(4-Nitrophenyl)-2-pyrrolidinmethyl]acrylat 97%](/deepweb/assets/sigmaaldrich/product/structures/194/557/0896d3d3-56d0-4b1e-9c08-1fdd246b5e86/640/0896d3d3-56d0-4b1e-9c08-1fdd246b5e86.png)