1479304
USP
Oseltamivir phosphate
United States Pharmacopeia (USP) Reference Standard
Synonyme(s) :
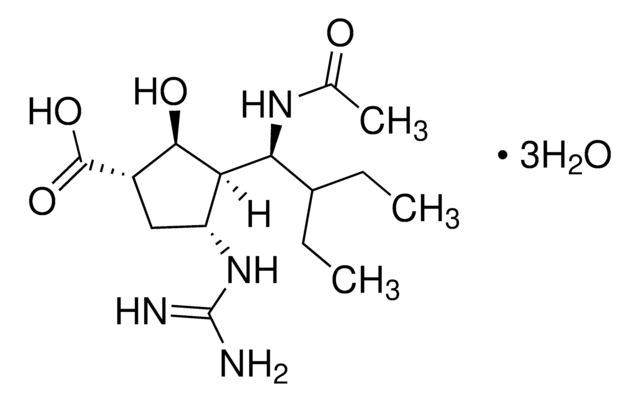
(3R,4R,5S)-4-Acetamido-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylic acid ethyl ester phosphate salt
About This Item
Produits recommandés
Qualité
pharmaceutical primary standard
Famille d'API
oseltamivir
Fabricant/nom de marque
USP
Application(s)
pharmaceutical (small molecule)
Format
neat
Chaîne SMILES
OP(O)(O)=O.CCOC(=O)C1=C[C@@H](OC(CC)CC)[C@H](NC(C)=O)[C@@H](N)C1
InChI
1S/C16H28N2O4.H3O4P/c1-5-12(6-2)22-14-9-11(16(20)21-7-3)8-13(17)15(14)18-10(4)19;1-5(2,3)4/h9,12-15H,5-8,17H2,1-4H3,(H,18,19);(H3,1,2,3,4)/t13-,14+,15+;/m0./s1
Clé InChI
PGZUMBJQJWIWGJ-ONAKXNSWSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- Neuraminidase Inhibition by Oseltamivir Phosphate: Oseltamivir phosphate′s mechanism as a neuraminidase inhibitor was highlighted in a study examining its effect on virus-neutralizing antibodies in influenza A-infected mice, demonstrating its impact on dosage and scheduling for effective flu treatment (Mikušová et al., 2022).
- Oseltamivir Phosphate in Lung Cancer Research: Research explored the use of oseltamivir phosphate loaded into pegylated-Eudragit nanoparticles for lung cancer therapy, focusing on characterization, prolonged release, cytotoxicity profile, apoptosis pathways, and the anti-angiogenic effect, thereby expanding its application beyond traditional antiviral uses (Yurtdaş-Kırımlıoğlu et al., 2022).
Remarque sur l'analyse
Autres remarques
Produit(s) apparenté(s)
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Aquatic Chronic 3 - Eye Irrit. 2 - Skin Sens. 1
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique